Sorafenib drug lipid nanosuspension and preparation method thereof
A nano-suspension, sorafenib technology, applied in the direction of drug combinations, pharmaceutical formulas, medical preparations of non-active ingredients, etc., can solve the problems of low oral bioavailability, poor water solubility, obvious absorption effects, etc., to achieve Good biocompatibility and safety, simple prescription, and improved absorption in the body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The screening of stabilizer in the preparation of embodiment 1 Sorafenib nanosuspension
[0063] The basic prescription of Sorafenib nano-suspension is: phospholipid concentration is 10mg / mL; drug-to-lipid ratio is 1:10; auxiliary stabilizer dosage is 1%; organic phase water phase volume ratio is 1:10; 5mL / min; magnetic stirring speed 800r / min; ice bath.
[0064] The basic prescription was optimized by single factor investigation, and the auxiliary stabilizer of Sorafenib lipid nanosuspension was screened. The particle size and particle size distribution were the main factors to be investigated, and its shape and uniformity were observed to examine its formulation and process. , and the results are shown in Table 1.
[0065] Table 1 The impact of the type of auxiliary stabilizer on the particle size and potential of Sorafenib lipid nanosuspension
[0066]
[0067] It can be seen from Table 1 that when Tween-80 is used as an auxiliary stabilizer, the obtained partic...
Embodiment 2
[0071] The preparation of embodiment 2 Sorafenib lipid nanosuspension--nanoprecipitation method
[0072] Weigh 9 mg of sorafenib and 75 mg of phospholipids in 1 mL of methanol as the methanol phase; dissolve Tween-80 in water to obtain a 1.5% (g / 100 mL) Tween solution as the water phase. Under the condition of magnetic stirring in an ice bath, the methanol phase was slowly added dropwise at a rate of 5mL / min into the 10mL water phase. After the dropwise addition, the methanol was evaporated to obtain a lipid nanosuspension of Sorafenib.
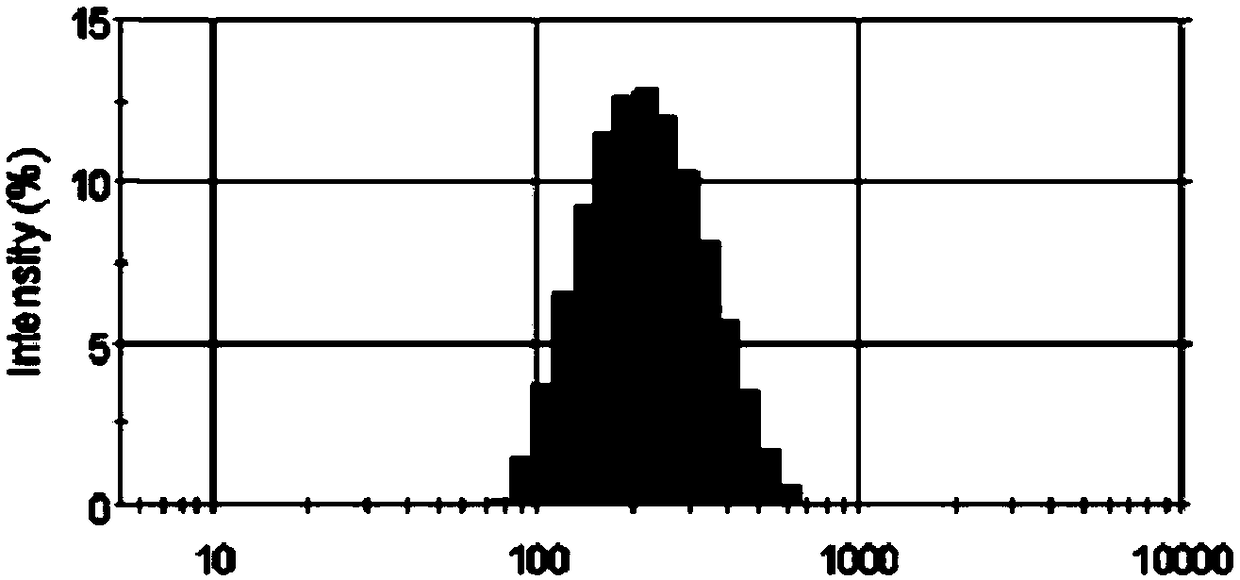
[0073] The above-mentioned prepared Sorafenib lipid nanosuspension has an average particle diameter of 164.5nm, a polydispersity coefficient of 0.202, a drug loading of 10.8%, potential-11.2mV, and a particle size distribution of figure 1 shown.
[0074] Get an appropriate amount of Sorafenib lipid nanosuspension prepared above, drop it on the copper grid, carry out negative staining with 2% phosphotungstic acid, observe under the transmissi...
Embodiment 3
[0075] Preparation of Example 3 Sorafenib Lipid Nanosuspension--High Pressure Homogenization
[0076] Accurately weigh 250 mg of phospholipids and Tween-80 and add them to 50 mL of distilled water (the concentration of Tween-80 is 1.0% (g / 100 mL)) to dissolve to form a dispersion medium (solution A). Add 25 mg of sorafenib and disperse uniformly by ultrasonic to obtain suspension B. Continue to use high-speed shearing machine 20000r / min high-speed shearing for 3min to prepare suspension C. Then suspension C is adopted high-pressure homogeneous method, circulates 5 times at 200bar respectively, 1000bar circulates 15 times, makes Sorafenib lipid nanosuspension, average particle diameter is 178.6nm, polydispersity coefficient 0.199, loaded The dose is 11.2%, and the potential is -10.2mV.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com