Beta-galactosidase two-site mutant with high transglycosylation and low hydrolytic activity and preparation method thereof
A technology of galactosidase and hydrolysis activity is applied in the field of preparation of β-galactosidase double point mutants with high transglycosidase activity and low hydrolysis activity, and can solve the problem of inability to Effectively take into account the influence of hydrolysis activity, low success rate, complicated operation and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] 1. Construction of E303C / F341S double point mutant
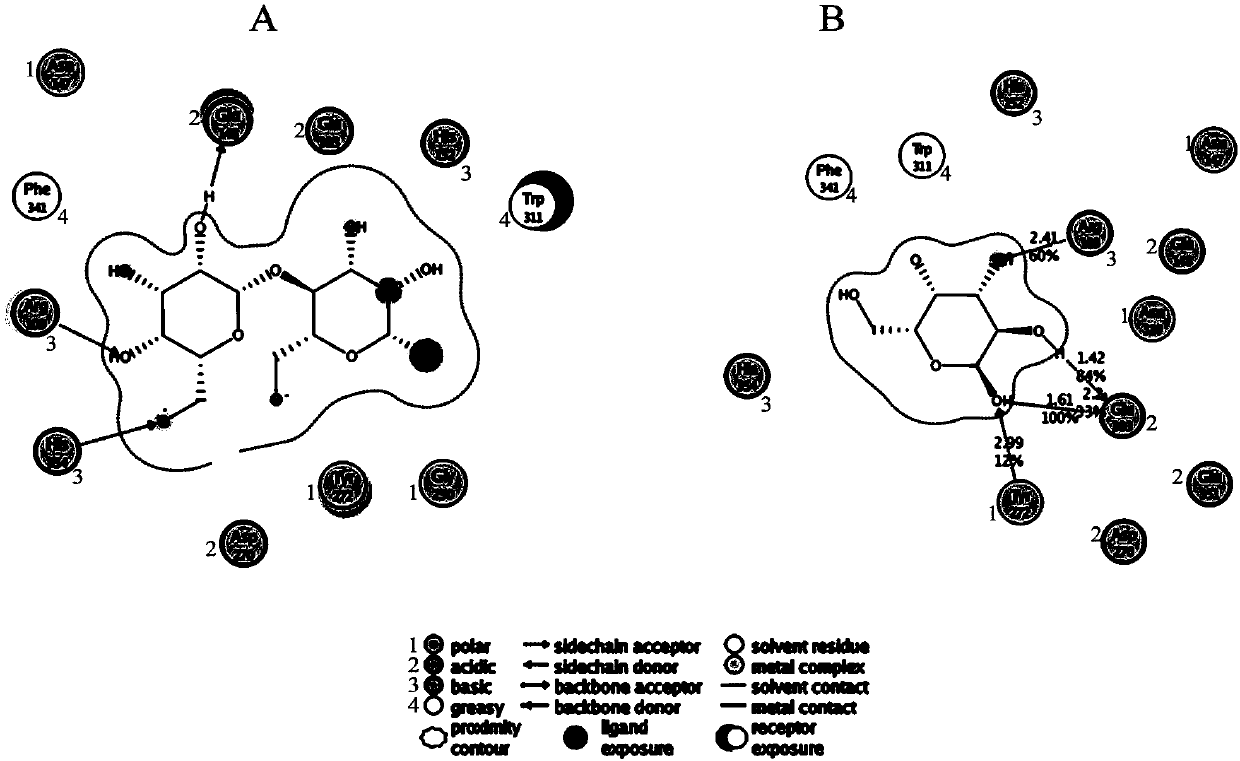
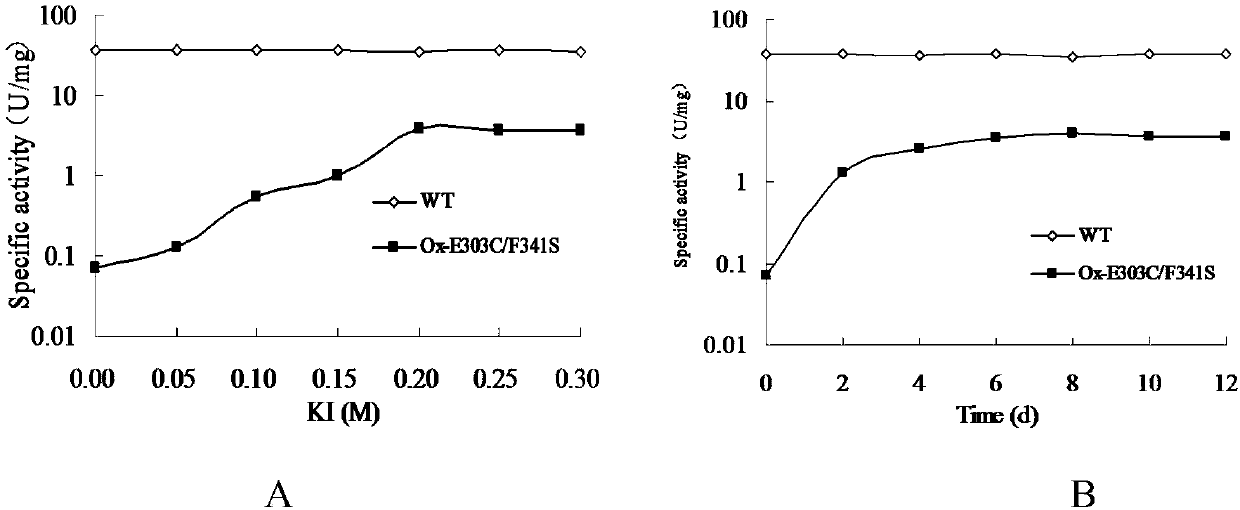
[0055] The amino acid sites involved in the substrate binding of BgaB, a thermostable β-galactosidase derived from Bacillus stearothermophilus, were predicted by molecular simulation and docking with galactose and substrates, and the substrate binding sites were verified and studied by point mutations. Regulatory function of enzyme catalytic activity. Among them, Glu148 and Glu303 are catalytic sites (such as figure 1 shown). In the non-catalytic site involved in substrate binding, the mutation of the Phe341 site can change the affinity of the enzyme to the hydrolysis product galactose, and regulate the inhibitory effect of the reaction product galactose on the enzyme activity. Existing studies have shown that catalytic amino acid sites and non-nucleophilic functional sites can regulate the transglycosidic activity of enzymes. In order to improve and develop the catalytic function of the enzyme for transglycosidat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com