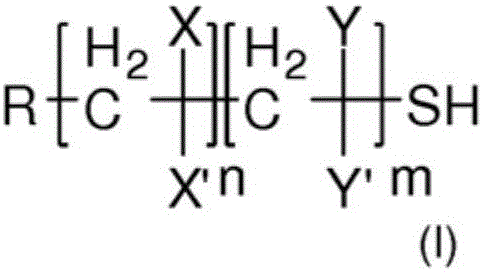Polyphosphorus polymer that is thiol-functionalised at the chain ends and production method thereof
A technology of polymers and polymer chains, applied in the field of polyphosphorus-based polymers, can solve the problems of polymer elastomer property degradation and performance degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
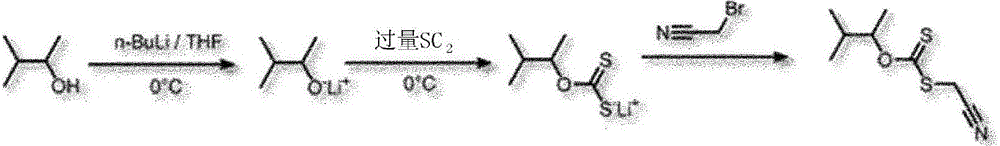
[0111] Example 1: Synthesis of xanthates with C4 cyanomethyl functional groups
[0112] - Reaction scheme
[0113]
[0114] Scheme 1. Synthesis of xanthates with C4 cyanomethyl functional groups
[0115] 6g (6.81x 10 -2mol) 3-methylbutanol was dissolved in 45ml THF. BuLi solution (1.6M in hexane) (46.5ml, 7.44x 10 -2 mol) was added dropwise to the reaction mixture at 0 °C. It was allowed to react with stirring for 30 minutes. Carbon disulfide (30ml, 4.96x 10 -1 mol) was added dropwise to the reaction medium at 0°C. The reaction mixture was then maintained at 0 °C for 30 minutes under magnetic stirring. Divide 11.6g (13.62x 10 -2 mol) bromoacetonitrile was added dropwise to the reaction mixture, and then the solution was kept under stirring for 15h. After evaporating THF, pass through CH 2 Cl 2 / water (1:1) to extract and purify the residue. Evaporate CH in vacuo 2 Cl 2 solution. After cleanup and evaporation on a chromatographic column (eluent: petroleum ethe...
Embodiment 2
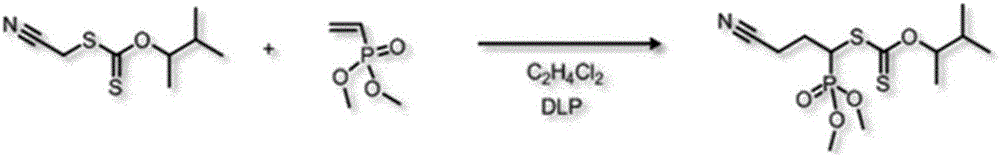
[0118] Example 2: Synthesis of dimethyl vinyl phosphonate DMVP-C4 monoadduct
[0119] - Reaction scheme
[0120]
[0121] Scheme 2. Synthesis of DMVP-C4 monoadduct
[0122] C4 xanthate (2.76g, 13.59x 10 -2 mol), dimethyl vinyl phosphonate (1g, 7.35x 10 - 3 mol) and 1,2-dichloroethane solvent (6ml) were introduced into a 25ml round bottom flask covered with a reflux condenser. The mixture was degassed under argon for 15 minutes. The reaction mixture was then maintained at the reflux point of the solvent (95° C.) for 7 hours with magnetic stirring. 5 mole % dilauroyl peroxide was added every 60 minutes up to 25 mole %. After cleanup on a chromatography column (eluent: ethyl acetate) and evaporation, the final synthesis yield was 65%.
[0123] 1 H NMR (300MHz, CDCl 3 , δ=ppm): 5.58 (1H, m, O-CHCH 3 ), 4.35 (1H, m, NC-CH 2 -CH 2 -CH 1 -S-C=S), 3.82(3H, s, P=(OCH 3 ) 2 ), 2.62 (2H, m, NC-CH 2 -CH 2 -CH 1 -S-C=S), 2.45-2.21 (2H, m, NC-CH 2 -CH 2 -CH 1 -S-C=S)...
Embodiment 4
[0132] Example 4: Pyrolysis of DMVP-C4 monoadduct
[0133] - Reaction scheme
[0134]
[0135] Scheme 4. Pyrolysis of DMVP-C4 monoadduct
[0136] DMVP-C4 monoadduct (250mg, 7.37x 10 -4 mol) and 1,2-dichlorobenzene solvent (3ml) were introduced into a 25ml round bottom flask covered with a reflux condenser. The reaction mixture was degassed under argon for 15 minutes and then kept in the dark at the reflux point of the solvent (200° C.) for 5 minutes. The pyrolysis yield was 70%.
[0137] 31 P NMR (300MHz, CDCl 3 , δ=ppm): 26.3 (1P, s, P=(OCH 3 ) 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com