Preparation method of magnetic KMS-1/Fe3O4 composite material and application of material for removing ciprofloxacin
A technology of ciprofloxacin and composite materials, which is applied in chemical instruments and methods, water/sludge/sewage treatment, alkali metal compounds, etc., to achieve the effects of high removal rate, low cost, and environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
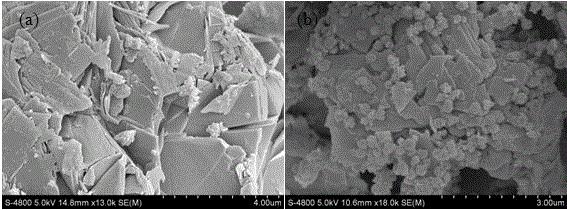
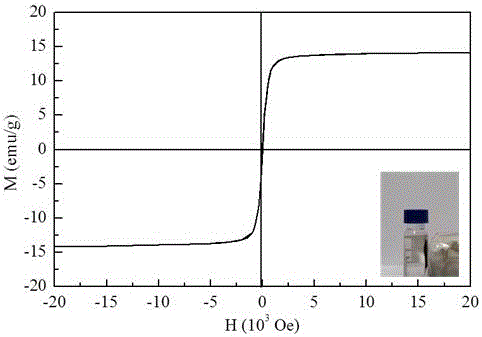
[0033] Example 1 : This KMS-1 / Fe 3 o 4 Composite material preparation and characterization, the details are as follows:
[0034] (1) The layered chalcogenide KMS-1 was synthesized according to the method reported in the literature ( JACS , 2009, 131: 6599–6607): Sn (23 mmol), Mn (11.53 mmol), K 2 CO 3 (11.53 mmol), S (69 mmol) and H 2 O (15.38 mL), hydrothermally reacted in an oven at 200°C for 4 days, cooled to room temperature, 2 Wash with ethanol several times in sequence, and finally dry it in a vacuum oven at 60°C before use;
[0035] (2) Nano Fe 3 o 4 Synthetic method, 4.05 g FeCl 3 ·H 2 O, 3 g PEG 2000, 10.8 g NaAC and 120 mL ethylene glycol were placed in a 200 mL reactor, and hydrothermally reacted in an oven at 200 °C for 20 h. After cooling to room temperature, wash with ethanol several times with the aid of a magnet, and finally dry it in a vacuum oven at 60°C before use;
[0036] (3) KMS-1 / Fe 3 o 4 The preparation method is as follows: take 0.1g-0...
Embodiment 2
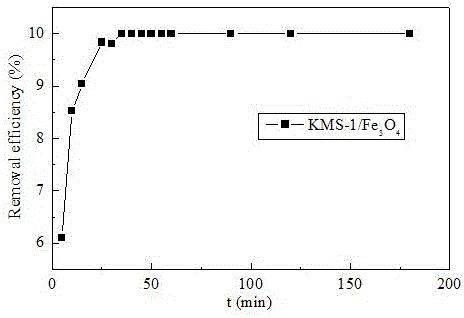
[0038] Example 2 : KMS-1 / Fe 3 o 4 Adsorption and removal of ciprofloxacin (CIP) in water under different reaction time conditions, the specific content is as follows:
[0039] (1) Prepare KMS-1 / Fe according to Example 1 3 o 4 composite materials;
[0040] (2) Use KMS-1 / Fe 3 o 4 Adsorb the CIP solution with an initial concentration of 10 mg / L, shake it in a shaker at 25°C at a speed of 300 r / min, and take samples at different time periods (10-180min). Then filter with filter paper, and the gained filtrate measures the concentration of CIP by high performance liquid chromatography;
[0041] (3) Draw the obtained data into a graph, such as image 3 As shown, when the reaction was carried out for 5 minutes, the equilibrium adsorption capacity of CIP was 6 mg / g. As the reaction time increased, the adsorption capacity of CIP also increased rapidly, and the adsorption was saturated at about 30 min, and the maximum adsorption capacity was 10 mg / g, continue to prolong the r...
Embodiment 3
[0042] Example 3 : initial pH vs. KMS-1 / Fe 3 o 4 Composite system adsorption removes the effect of ciprofloxacin (CIP) in water, the specific content is as follows:
[0043] (1) Prepare KMS-1 / Fe according to Example 1 3 o 4 composite materials;
[0044] (2) Prepare a solution with a concentration of 10 mg / LCIP, measure 20 mL of CIP target solution into 12 clean reaction bottles, and adjust the pH of the target solution to 0.86, 2.0, 3.05, 4.1, 5.09, 6.07, 7.0, 8.0, 9.0, 10.0, 11.0, and 12.0. Add a certain amount of KMS-1 / Fe to 12 reaction bottles respectively 3 o 4 Composite material, stirred at room temperature (300 rpm) for 3 hours, then sampled and analyzed;
[0045] (3) Draw the obtained data into a graph, such as Figure 4 As shown, when the pH is 1, the maximum adsorption capacity is 10 mg / g, when the pH = 2, the maximum adsorption capacity is slightly decreased, and in the range of pH 3-8, the adsorption capacity remains at 9 mg / g Above, when the pH was 9, t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com