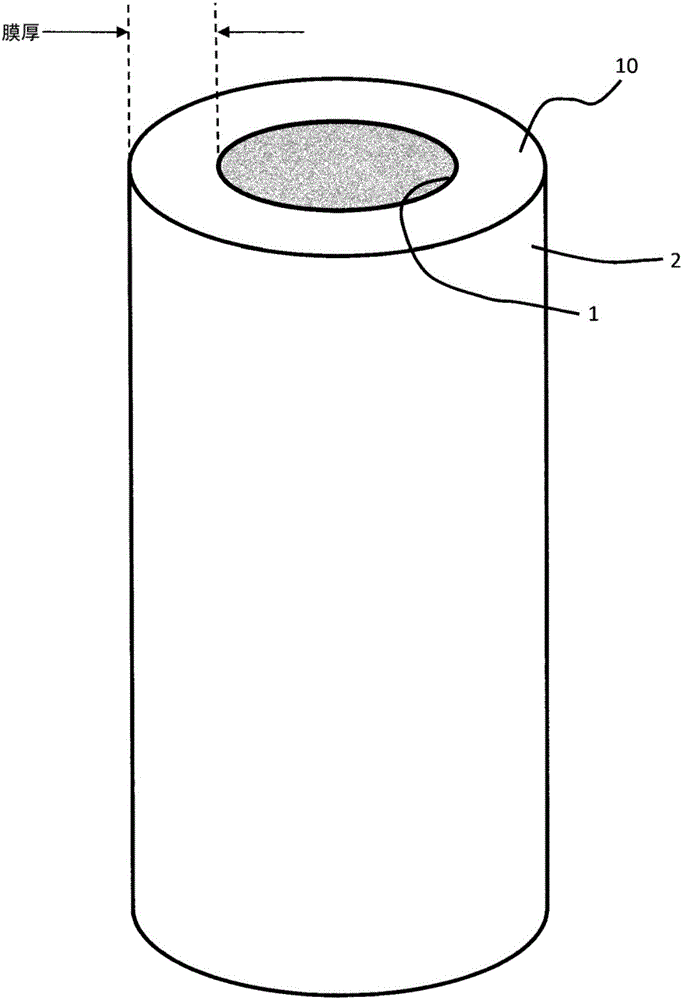
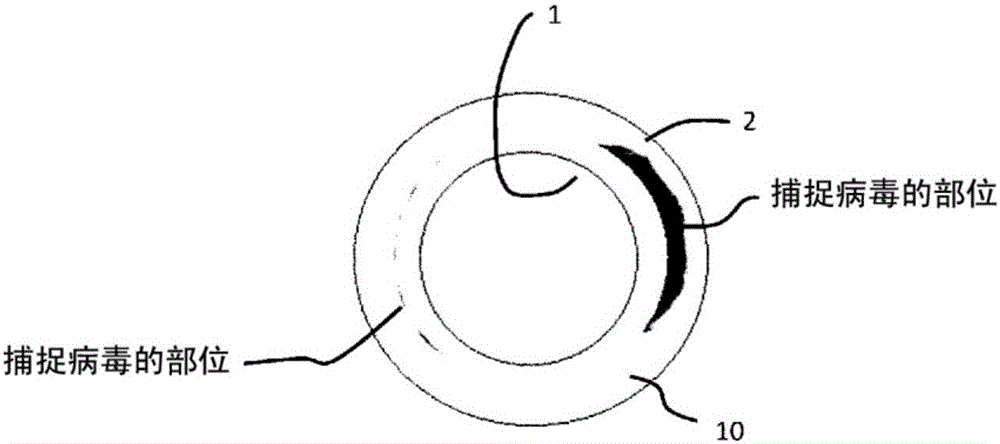
Virus removal membrane
A virus and removal rate technology, applied in membranes, membrane technology, semi-permeable membrane separation, etc., can solve the problems of lack of filtration efficiency, filtration speed, and insufficient research on the conditions for virus removal, so as to achieve high filtration efficiency and virus removal. High removal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0077] (production of membrane to remove virus)
[0078] Cotton linters (average molecular weight 1.44×10 5) and sodium sulfate (KISHIDA CHEMICAL Co., Ltd.) were dissolved in a cuproammonia solution produced by a known method, and filtered to defoam, the concentration of cellulose and the concentration of sodium sulfate as an inorganic salt were as follows Figure 5 and Figure 6 As shown, a spinning stock solution having an ammonia concentration of 4.4% by weight and a copper concentration of 2.7% by weight was obtained. Then, the spinning dope is discharged from the outer spinning outlet of the double-layer ring spinning outlet at 3.0cc / min or 3.65cc / min, and simultaneously the Figure 5 and Figure 6 The internal coagulation liquid of acetone / ammonia / water in the weight ratio shown was discharged at 1.8 cc / min from the central spinning outlet of the double-layer annular spinning nozzle.
[0079] The spinning dope and internal coagulation liquid discharged from the doubl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com