Method for producing harmful-organism control agent, and intermediate thereof
A compound and general formula technology, applied in the preparation of hydrogenated polysulfide/polysulfide, sulfide preparation, organic chemistry, etc., can solve the problem of using a large number of high-valent halogenated alkylating agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
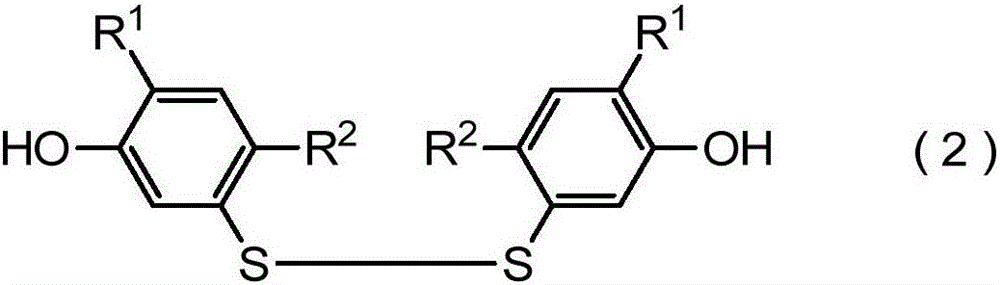
[0598] Production of bis(2,4-dimethyl-5-hydroxyphenyl) disulfide
[0599]
[0600] To the reaction flask were added 44.32 g (28.0 mmol) of 5-mercapto-2,4-dimethylphenol, 42 mg (0.28 mmol) of sodium iodide, and 80 mL of ethyl acetate. While stirring the mixture at room temperature, 1.59 g (14 mmol) of 30% aqueous hydrogen peroxide was slowly added dropwise thereto. The mixture was stirred at room temperature for 1 hour. 5 mL of saturated aqueous sodium sulfite solution was added to the reaction mixture, and the mixture was stirred at room temperature for 10 minutes, then concentrated hydrochloric acid was added thereto to adjust the pH of the aqueous layer to about pH 1-2. The obtained mixture was partitioned into an organic layer and an aqueous layer, and the organic layer was separated. The organic layer was dried over anhydrous magnesium sulfate, and after filtration, the solvent was distilled off under reduced pressure. The obtained residue was purified by silica gel ...
Embodiment 2
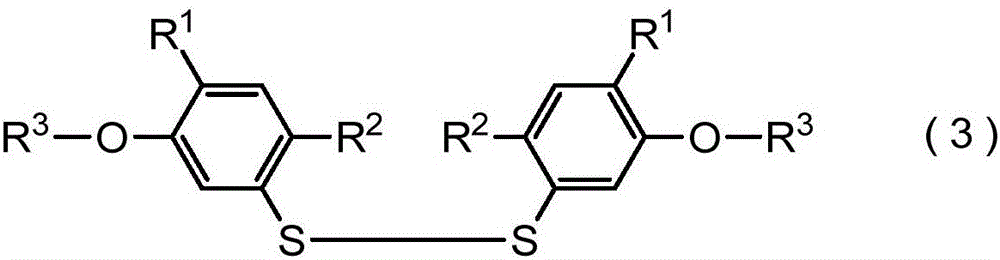
[0604] Production of bis[2,4-dimethyl-5-(6-trifluoromethylthiohexyloxy)phenyl]disulfide
[0605]
[0606] In the reaction flask, add bis(2,4-dimethyl-5-hydroxyphenyl) disulfide 2.60g (8.5mmol), 1-bromo-6-trifluoromethylthiohexane 4.73g (17.9 mmol), potassium carbonate 2.58g (18.7mmol), tetrabutylammonium iodide 0.63g (1.7mmol) and N,N-dimethylformamide 17mL. The mixture was stirred at 80° C. for 20 hours under nitrogen atmosphere. Dilute hydrochloric acid, water and diethyl ether were added to the reaction mixture, the mixture was partitioned into an organic layer and an aqueous layer, and the organic layer was separated. The organic layer was dried over anhydrous magnesium sulfate, filtered, and the solvent was distilled off under reduced pressure. The obtained residue was purified by silica gel column chromatography to obtain 4.95 g of bis[2,4-dimethyl-5-(6-trifluoromethylthiohexyloxy)phenyl] disulfide, yield 86 %.
[0607] 1 H‐NMR (300MHz, CDCl 3 )δ(ppm): 6.92(s,2H...
Embodiment 3
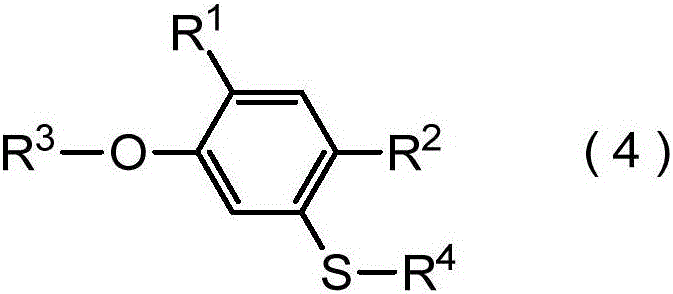
[0609] Production of 6-trifluoromethylthiohexyl-[2,4-dimethyl-5-(2,2,2-trifluoroethylthio)phenyl]ether
[0610]
[0611] Add bis[2,4-dimethyl-5-(6-trifluoromethylthiohexyloxy)phenyl]disulfide 337.4mg (0.50mmol), p-toluenesulfonic acid 2,2, 266.9 mg (1.05 mmol) of 2-trifluoroethyl ester, 152.0 mg (1.10 mmol) of potassium carbonate, 231.2 mg (1.50 mmol) of Rongalite (trade name) (sodium formaldehyde sulfoxylate dihydrate) and N,N-dimethyl 2 mL of methyl formamide. The mixture was stirred at 50 °C under nitrogen atmosphere for 16 hours. Dilute hydrochloric acid, water and diethyl ether were added to the reaction mixture, the mixture was partitioned into an organic layer and an aqueous layer, and the organic layer was separated. The organic layer was dried over anhydrous magnesium sulfate, and after filtration, the solvent was distilled off under reduced pressure. The obtained residue was purified by silica gel column chromatography to obtain 242.0 mg of 6-trifluoromethylthi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com