Small-molecular micelle drug-loaded nano-system, as well as preparation method and application thereof
A nano drug-loading and drug-loading system technology, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of poor stability and low loading rate of small molecule micelles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1 Preparation of cross-linked small molecule micelles nano-drug loading system.
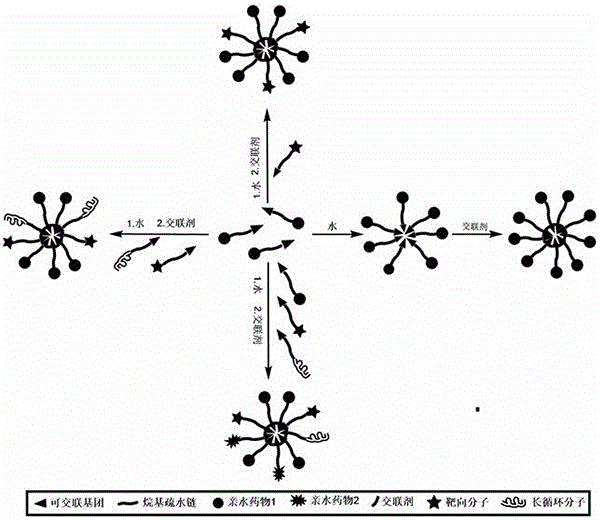
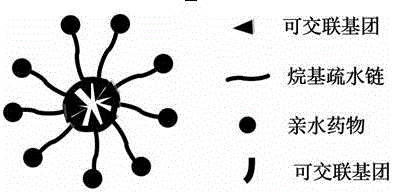
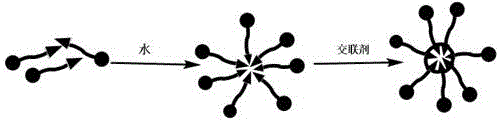
[0071] a kind of like figure 2 In the small molecule micelle nano drug loading system, L1 is an amphiphilic molecule, the hydrophobic end is a cross-linkable alkyl chain, and the hydrophilic end is a hydrophilic drug. We use lipoic acid at the hydrophobic end, and jecitabine at the hydrophilic end. L1 self-assembles to form micelles through the amphiphilic principle, and then forms a stable cross-linked micellar drug-loading system through internal cross-linking.
[0072] (1) The following is the synthetic route of L1:
[0073]
[0074] resolve resolution:
[0075] The raw materials are Jecitabine, lipoic acid and organic base N,N - Diisopropylethylamine (DIPEA), condensing agent 1-ethyl-(3-dimethylaminopropyl)carbodiimide (EDCI), solvent DMF. Combine jesitabine and N,N -Diisopropylethylamine was dissolved in DMF, and then lipoic acid and 1-ethyl-(3-dimethylaminopropyl) ...
Embodiment 2
[0089] Example 2 Stability testing of small molecule cross-linked micellar nano-drug loading system.
[0090] Take 5 mL of water in 10 mL vials and mark them as No. 1 and No. 2 bottles respectively. Add 25 μL of L1 (0.1 mmol / ml ) solution respectively under the condition of vigorous stirring of water, and finally a micellar solution with a concentration of 0.5 mmol / L can be obtained. According to the previous method, the micelles in the No. 1 vial were cross-linked according to the method of Example 1 (4), and L1 cross-linked micelles and L1 uncross-linked micelles were finally prepared respectively. 4.5 mL of L1 crosslinked micelles and L1 uncrosslinked micelles were incubated with 0.5 mL of fetal bovine serum, and the DLS values of the two micelles were measured at different time points. The result is as Figure 5 As shown in (a), after the L1 cross-linked micelles and L1 uncross-linked micelles were incubated in serum for 1 h, the particle size changes before and after ...
Embodiment 3
[0091] Example 3 In vitro release of cross-linked small-molecule micellar nano-drug delivery system (enzyme-sensitive detection)
[0092] In vitro release experiments of nanomicelles: L1 cross-linked micelles and L1 uncross-linked micelles were prepared at a concentration of 0.5 mmol / L, and the pH of the solution was adjusted to 5.5. Take 5 mL of materials respectively, place them in a water bath shaker at 37°C, add 40 μL of Cathepsin B (5 U / mL) or no Cathepsin B, shake and incubate, and sample 100 μL at the set time point, The samples taken out were detected by high performance liquid chromatography to detect the content of the drug jecitabine released in each sample, and finally the release curves of L1 cross-linked micelles and L1 uncross-linked micelles in the presence or absence of protease B were made , the result is as Figure 6 shown. It can be seen from the figure that the nanoparticles are hardly released in the absence of protease B (as a comparison, the present e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com