A method and system for producing hydrocarbons from methanol
A technology for producing hydrocarbons and methanol from methanol, which is applied to chemical instruments and methods, producing hydrocarbons from oxygen-containing organic compounds, and production of bulk chemicals, etc. The effect of reducing electricity consumption, large heat transfer coefficient and increasing steam output
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
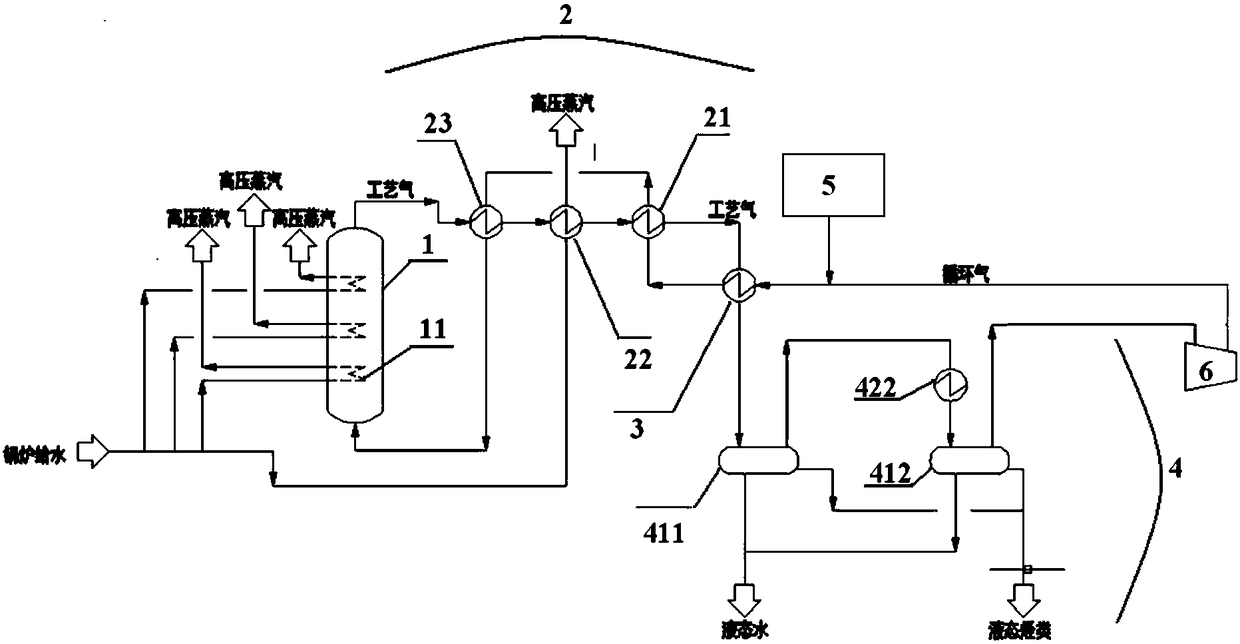
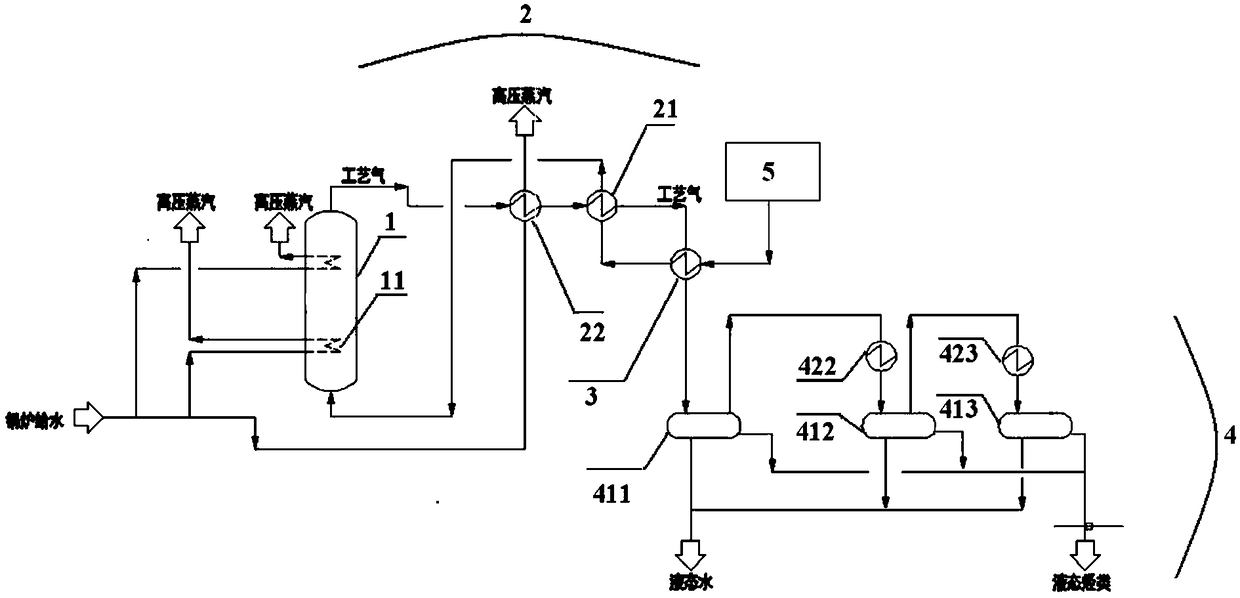
[0060] According to another specific embodiment of the present invention, the heat exchange device includes a first preheater, a waste heat boiler, and a second preheater, and the heat exchange process of the heat exchange device includes: The gasified methanol-containing raw material passes through the first heat exchanger and the second heat exchanger successively, and the hydrocarbon-containing process gas generated by the reaction in the fluidized bed reactor passes through the second preheater, waste heat boiler and first preheater successively. Heater, that is, the gasified methanol-containing feedstock produced by the gasifier is heat-exchanged with the process gas from the waste heat boiler in the first preheater; The first superheated methanol-containing raw material obtained after heat exchange in the preheater and the process gas from the fluidized bed reactor are heat exchanged in the second preheater. Wherein, the second superheated methanol-containing raw materia...
Embodiment 1
[0097] This embodiment is used to illustrate the methanol-to-hydrocarbon method and system provided by the present invention
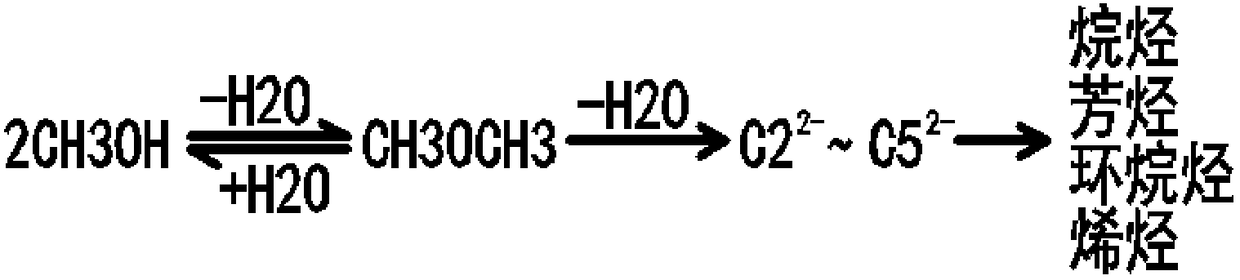
[0098] Such as figure 1 As shown, fresh methanol liquid at room temperature is sent from the methanol supply device 5 to the pipeline leading to the vaporizer 3, and the mass flow rate of the returned circulating gas is 2.5 times that of methanol. After the liquid methanol and the circulating gas are mixed in the pipeline, they enter the gasifier 3, exchange heat with the hydrocarbon-containing process gas, and then completely vaporize, and the temperature rises to 80.5°C. The vaporized gas enters the primary preheater 21 and the secondary preheater 23 respectively, and performs indirect heat exchange in spaces independent of the process gas. After the vaporized methanol-containing gas is heated twice, The temperature is raised to 280°C, and it enters from the bottom of the fluidized bed reactor 1 to flow upward and react. The reaction pressure is 0....
Embodiment 2
[0103] This embodiment is used to illustrate the methanol-to-hydrocarbon method and system provided by the present invention
[0104] use figure 1 Shown equipment and carry out methanol to hydrocarbon according to the method for embodiment 1, difference is,
[0105] (1) Methanol is mixed with the circulation gas of 1.5 times of mass flow rate;
[0106] (2) After heating twice, the methanol-containing gas is heated to 250°C;
[0107] (3) The reaction pressure is 0.6MPa, and the three-stage reaction temperatures are 400°C, 390°C, and 380°C respectively along the flow direction of methanol;
[0108] (4) The temperatures of the hydrocarbon-containing process gas after three times of cooling are 360°C, 300°C, and 130°C respectively.
[0109] Record the steam consumption, steam production and electricity consumption in the whole process, and the results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com