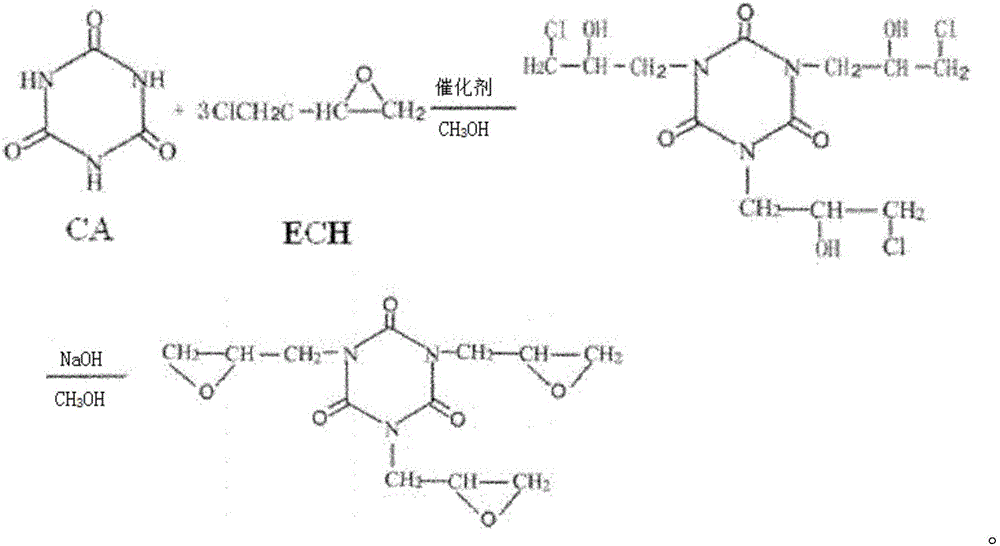
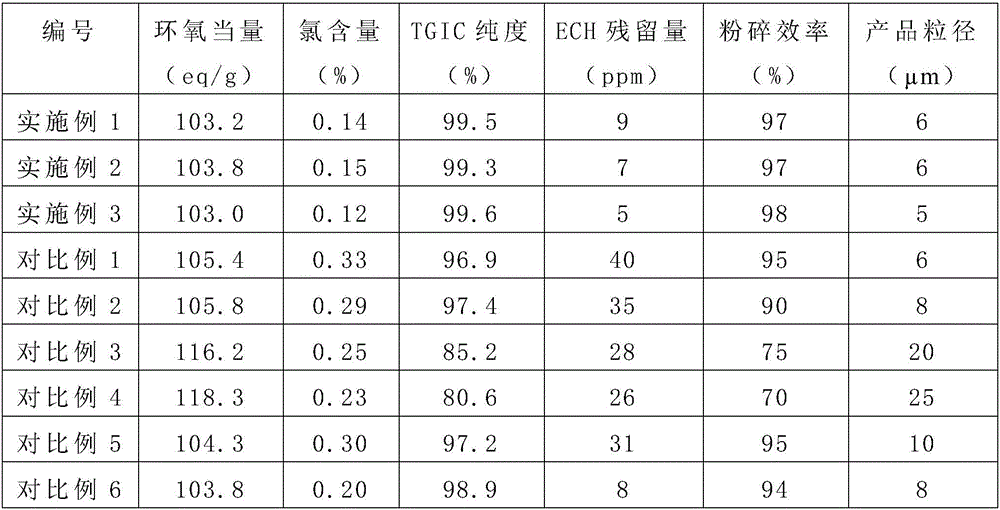
Method for preparing electronic grade triglycidyl isocyanurate
A technology of triglycidyl isocyanurate, applied in the direction of organic chemistry, can solve problems such as endangering the physical and mental health of workers, affecting product yield and quality, and complicated distillation steps, so as to avoid a large number of direct contact and recycling Simple and easy, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The method for preparing described TGIC product in the present embodiment comprises the steps:
[0029] (1) Add 200Kg of cyanuric acid, 1003Kg of epichlorohydrin, 20Kg of benzyltriethylammonium chloride, and 20Kg of methanol into the reaction kettle and mix well, and heat the mixture system to 110°C, and Carry out the reaction for about 5 hours until the mixed solution gradually becomes clear and transparent, and then carry out vacuum distillation to remove unreacted ECH according to the conventional method in the prior art;
[0030] (2) Transfer the synthesis solution after the reaction in step (1) to the cyclization kettle, add 1000Kg of methanol solvent and mix evenly, and add 200Kg of caustic soda in 4 times, control the reaction temperature at 20°C for 110min; cyclization After the reaction is over, centrifuge and perform solid-liquid separation to obtain a solid mixture of TGIC products and salts and methanol mother liquor;
[0031] (3) Collect the solid mixture ...
Embodiment 2
[0033] The method for preparing described TGIC product in the present embodiment comprises the steps:
[0034] (1) Add 200Kg of cyanuric acid, 574Kg of epichlorohydrin, 16Kg of benzyltriethylammonium chloride, and 10Kg of methanol into the reaction kettle and mix well, and heat the mixture system to 90°C, and Carry out the reaction for about 3 hours until the mixed solution gradually becomes clear and transparent, and then carry out vacuum distillation to remove unreacted ECH;
[0035](2) Transfer the synthesis solution after the reaction in step (1) to the cyclization kettle, add 1600Kg of methanol solvent and mix evenly, and add 217Kg of caustic soda in 3 times, control the reaction temperature at 25°C for 120min; cyclization After the reaction is over, centrifuge and perform solid-liquid separation to obtain a solid mixture of TGIC products and salts and methanol mother liquor;
[0036] (3) Collect the solid mixture after solid-liquid separation, add 2070Kg of water to was...
Embodiment 3
[0038] The method for preparing described TGIC product in the present embodiment comprises the steps:
[0039] (1) Add 200Kg of cyanuric acid, 717Kg of epichlorohydrin, 8Kg of benzyltriethylammonium chloride, and 10Kg of methanol into the reaction kettle and mix well, and heat the mixture system to 100°C, and Carry out the reaction for about 4 hours until the mixed solution gradually becomes clear and transparent, and then carry out vacuum distillation to remove unreacted ECH;
[0040] (2) Transfer the synthetic solution after the reaction in step (1) to the cyclization kettle, add 1000Kg of methanol solvent and mix well, and add 186Kg of caustic soda in 4 times, control the reaction temperature at 15°C for 90min; cyclization After the reaction is over, centrifuge and perform solid-liquid separation to obtain a solid mixture of TGIC products and salts and methanol mother liquor;
[0041] (3) Collect the solid mixture after solid-liquid separation, add 1655Kg of water to wash ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com