Enzyme, encoding gene thereof, application of enzyme and encoding gene, and method for preparing ginseng saponin compound K
A compound and gene technology, applied in the field of genetic engineering, can solve problems such as low conversion rate and unsatisfactory
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] This example is used to illustrate the preparation of the enzyme provided by the present invention.
[0067] (1) Acquisition of genes
[0068] According to the nucleotide sequence shown in SEQ ID NO: 1, the corresponding gene was obtained by artificial chemical synthesis (entrusted to Kunming Shuoqing Biotechnology Co., Ltd.).
[0069] (2) Construction of expression vectors and recombinant strains
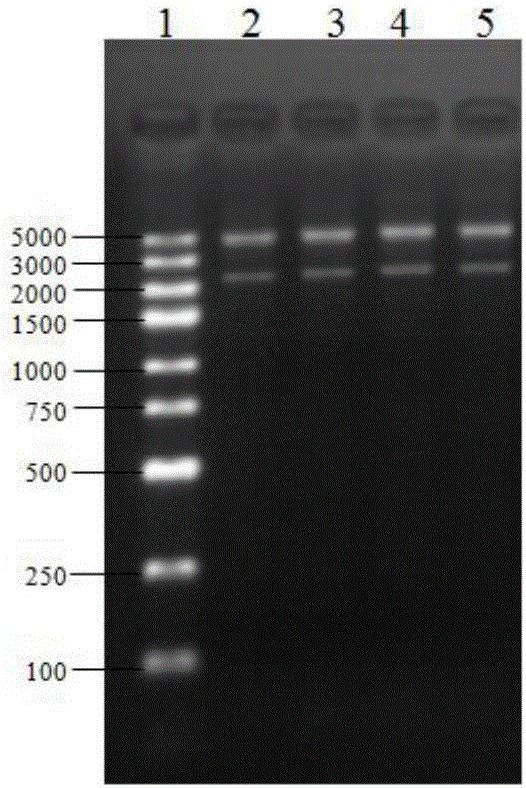
[0070] Both the gene obtained in step (1) and the Pichia pastoris expression vector pPICZɑA (purchased from Invitrogen, USA) were digested with EcoR I and Xba I, and then T4 ligase (purchased from Bao Biological Engineering (Dalian) Co., Ltd. ) to connect the above two enzyme-digested products to obtain a recombinant plasmid, and the double-enzyme digestion verification result of the recombinant plasmid is as follows: figure 1 As shown, wherein, 1 is a DNA marker, and 2-5 are recombinant plasmids.
[0071] The obtained recombinant plasmid was transformed into Pichia pasto...
Embodiment 2
[0079] This example is used to illustrate the application of the enzyme provided by the present invention in the preparation of ginsenoside CK.
[0080] Get 2mL by the purified enzyme liquid that embodiment 1 obtains, add ginsenoside Rb 3 Pure product (reported by Yunnan Yunuo Bioengineering Co., Ltd. (Li Haizhou, Zhang Yingjun, Yang Chongren. Further research on the chemical constituents of Panax notoginseng. Natural Product Research and Development, 2006, 18(4): 549-554) Methods Separation and purification from the total saponins of Panax notoginseng; Content: HPLC≥99%; Molecular weight, 1 H and 13 The chemical shift value of C NMR is consistent with that reported in the literature), so that the final concentration is 1.0 mg / mL, and it is hydrolyzed at 45 °C and pH 4.5 for 48 hours, and the treated sample is taken for TLC analysis. The enzyme solution can hydrolyze Ginsenoside Rb 3 For ginsenoside CK (such as Figure 4 Shown in 1, 2 and 4, wherein, 1 is the pure product ...
Embodiment 3
[0083] This example is used to illustrate the application of the enzyme provided by the present invention in the preparation of ginsenoside CK.
[0084] Get 2 mL of the purified enzyme solution obtained in Example 1, add gypenoside IX (Gypenoside IX, Gyp IX) pure product (Yunnan Yunuo Bioengineering Co., Ltd. with reference to literature (Li Haizhou, Zhang Yingjun, Yang Chongren. Notoginseng Further research. Natural product research and development, 2006, 18 (4): 549-554) reported method is separated and purified from Panax notoginseng saponins; Content: HPLC≥99%; Molecular weight, 1 H and 13 The chemical shift value of C NMR is consistent with that reported in the literature), so that the final concentration is 1.0 mg / mL, and it is hydrolyzed at 45 °C and pH 4.5 for 48 hours, and the treated sample is taken for TLC analysis. The enzyme solution can hydrolyze Gypenoside IX is ginsenoside CK (such as Figure 4 Shown in 1, 3 and 5, wherein, 1 is ginsenoside CK pure product co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com