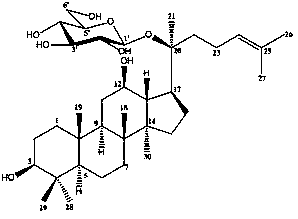
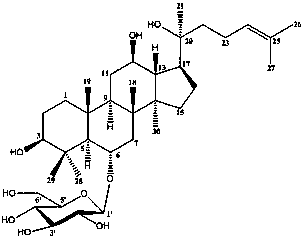
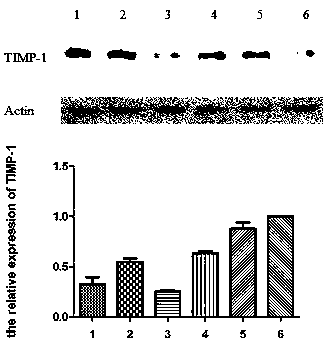
Application of ginsenoside ck, rh1 and their compositions in the preparation of drugs for improving non-alcoholic fatty liver fibrosis and insulin resistance
A technology for fatty liver fibrosis and insulin resistance, applied in drug combination, digestive system, pharmaceutical formula, etc., to achieve the effect of improving liver fibrosis, improving insulin resistance, and reducing cell steatosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: Ginsenoside CK, Rh 1 and CK:Rh 1 In vitro study of =1:1 regulation of gene expression related to collagen synthesis and degradation in rat hepatic stellate cells HSC-T6
[0035] (1) Materials and methods
[0036] 1. Cell line: Rat hepatic stellate cell line HSC-T6 was purchased from the cell bank of Kunming Institute of Zoology, Chinese Academy of Sciences. The phenotype is activated HSC with fibrosis characteristics. HSC-T6 cell lines were incubated with DMEM medium containing 100 U / mL penicillin, 100 μg / mL streptomycin and 10% heat-inactivated fetal calf serum at 37°C with a volume fraction of 5% CO 2 Culture in an incubator, replace the medium after 48 hours, digest with 0.25% trypsin when the cells grow to 80% density, passage once every 2~3 days, and use the logarithmic growth phase cells in the experiment.
[0037] 2. Ginsenoside CK, Rh 1 and CK:Rh 1 =1:1 effect on apoptosis of HSC-T6 cells
[0038] Take HSC-T6 in the logarithmic growth phase, ...
Embodiment 2
[0046] Embodiment 2: Ginsenoside CK, Rh 1 and CK:Rh 1 Effect of =1:1 on nonalcoholic fatty liver fibrosis and insulin resistance in rats
[0047] (1) Materials and methods
[0048] 1. Experimental animals
[0049] Clean-grade healthy male SD rats, 5 weeks old, weighing 160±10g, were provided by the Animal Department of Kunming Medical University, and the animal qualification certificate number: SCXK (Dian) 2005-2008. Raised in separate cages under the condition of no special pathogenic bacteria (SPF), controlled room temperature (23±1ºC), humidity 40%, 12 hours in light and dark, free to forage for food and drink water.
[0050] 2. Preparation of main reagents
[0051] High-fat feed: cholesterol (1%) + bile salt (0.3%) + propylthiouracil (0.1%) + lard (10%) + egg yolk (10%) + cornmeal (78.6%); common feed: for The standard feed for experimental animals was prepared by the Animal Department of Kunming Medical University, with an energy density of 14.56 kJ / g.
[0052] 3. M...
Embodiment 3
[0124] Embodiment 3: Ginsenoside CK, Rh 1 and CK:Rh 1 =1:1 preparation of dispersible tablets
[0125] Weigh ginsenoside CK: 200 g, Rh 1 : 200 g or CK:Rh 1 =1:1, put 200 g in a 50 L round bottom flask, add 20 L of absolute ethanol, and stir until completely dissolved. Add 600 g of medicinal oral grade soybean lecithin, stir until completely dissolved. Raise the temperature to 40°C, reflux and stir for 2 hours, and evaporate to dry ethanol to obtain ginsenoside CK-phospholipid complex, Rh 1 - Phospholipid complex or CK:Rh 1 =1:1-phospholipid complex.
[0126] Weigh 100 g of ginsenoside CK-phospholipid complex and Rh 1 - Phospholipid complex 100 g or CK:Rh 1 =1:1-phospholipid complex 100 g, microcrystalline cellulose 150 g, hydroxypropyl cellulose 16 g, cross-linked povidone 16 g through a 50-mesh sieve and mix well, then add 0.5 g Tween 80 for binding The agent was granulated through a 20-mesh sieve, dried at 55°C for 3 hours, then granulated through a 18-mesh sieve, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com