Ropivacaine mesylate freeze-dried powder injection for injection and preparation method thereof
A technology of ropivacaine mesylate and freeze-dried powder injection, which is applied in the field of preparation of ropivacaine mesylate freeze-dried powder injection for injection, and can solve the problem of unsuitability for large-scale industrial production, high cost input and complicated operation and other problems, to achieve the effect of good resolubility and clarity, high yield and good resolubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] A ropivacaine mesylate freeze-dried powder for injection, comprising the following components:
[0044] Ropivacaine mesylate 89.4g;
[0045] Mannitol 50g;
[0046] Add 5% ethanol aqueous solution to 1000mL;
[0047] Its preparation method is as follows:
[0048](1) Accurately weigh the ropivacaine mesylate of the prescription amount, add mass percentage concentration and be 5% ethanol solution, stir and dissolve; add the mannitol of recipe amount again, continue to stir and dissolve; Obtain mixed solution, wherein stir and dissolve When, the speed of stirring is 80 rpm;
[0049] (2) Regulate the pH value of the mixed solution obtained in step (1) to 4.0-6.0 with 0.1mol / L NaOH solution, and add an aqueous ethanol solution with a mass percentage concentration of 5% to the prescription amount;
[0050] (3) Add activated carbon for needles to the mixed solution obtained in step (2), the dosage of activated carbon for needles is 0.1% g / mL; after stirring and absorbing fo...
Embodiment 2
[0053] A ropivacaine mesylate freeze-dried powder for injection is characterized in that the freeze-dried powder includes the following components:
[0054] Ropivacaine mesylate 119.2g;
[0055] Mannitol 40g;
[0056] Add 10% ethanol aqueous solution to 1000mL;
[0057] Its preparation method is as follows:
[0058] (1) Accurately weigh the ropivacaine mesylate of the prescription amount, add the mass percent concentration and be 10% ethanol solution, stir and dissolve; add the mannitol of the recipe amount again, continue to stir and dissolve; obtain the mixed solution, the speed of stirring 120 rpm;
[0059] (2)) Use 0.1mol / L NaOH solution to adjust the pH value of the mixed solution obtained in step (1) to 4.0-6.0, and add an aqueous ethanol solution with a mass percentage concentration of 10% to the prescription amount;
[0060] (3) Add activated carbon for needles to the mixed solution obtained in step (2), the dosage of activated carbon for needles is 0.1% g / mL; afte...
Embodiment 3
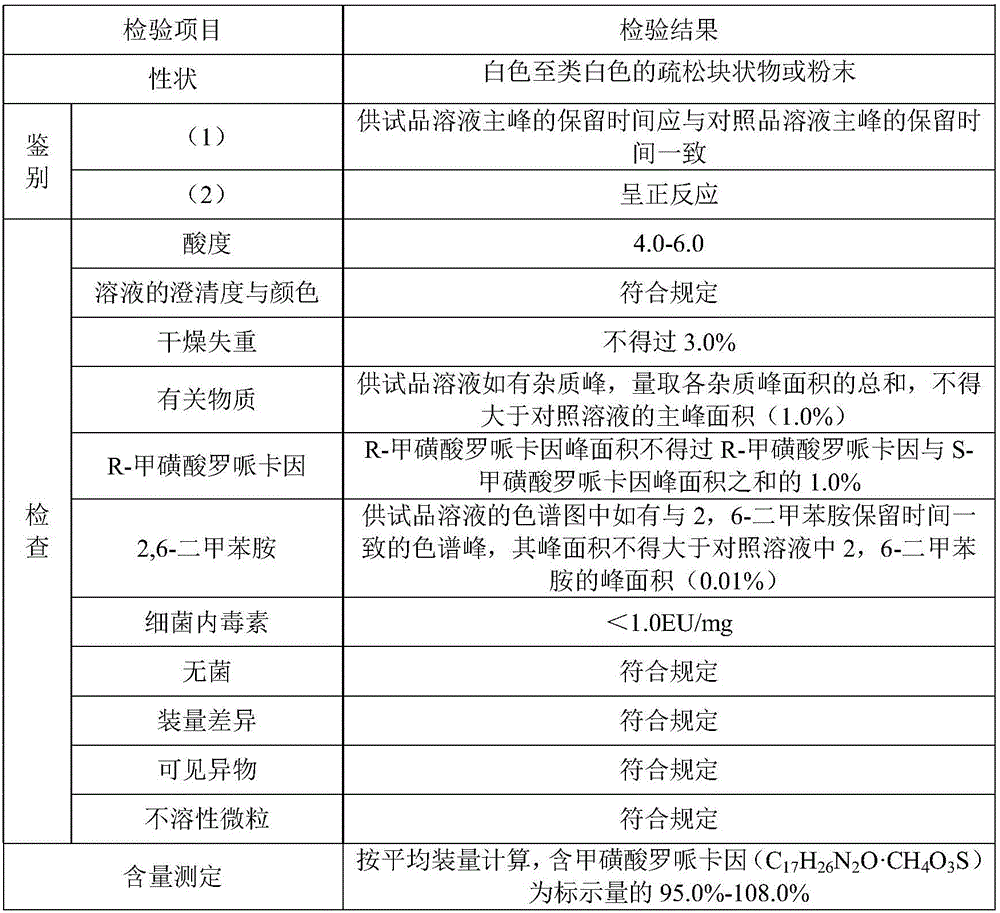
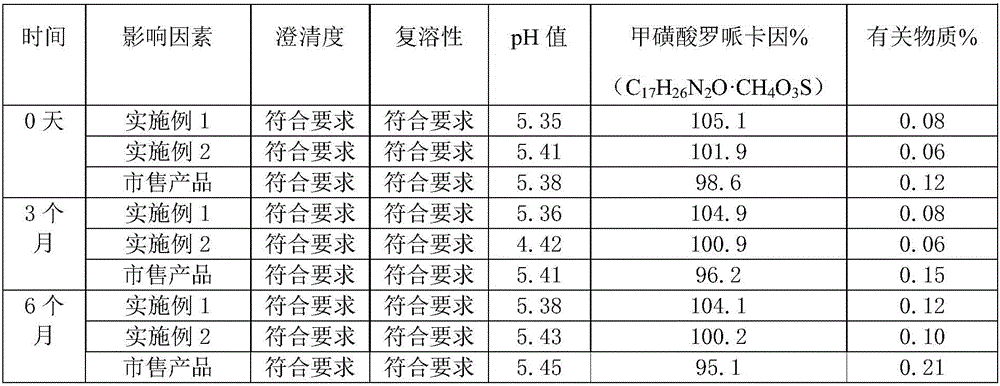
[0063] The sample obtained in Example 1 of the present application is detected, and the obtained results are as follows:
[0064]
[0065] As can be seen from the above table, the ropivacaine mesylate freeze-dried powder injection prepared by the present application fully complies with the relevant regulations of the Pharmacopoeia.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com