Catalyst for catalytic oxidation of carbonic oxide and preparation method of catalyst
A carbon monoxide, catalytic oxidation technology, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve the problem of low activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
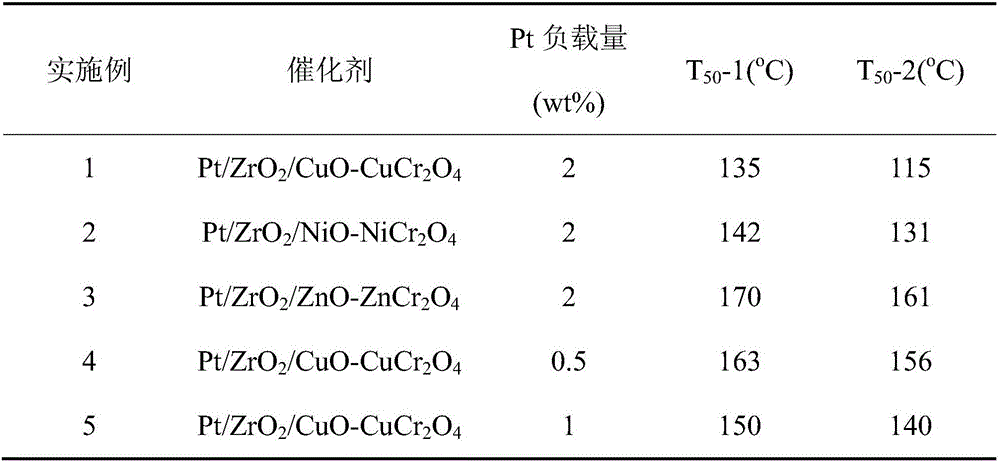
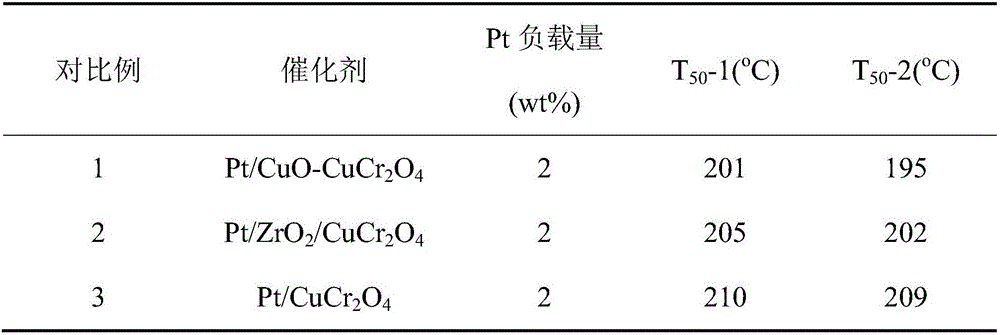
Embodiment 1
[0018] (1) According to Cu(NO 3 ) 2 ·3H 2 O, Cr(NO 3 ) 3 9H 2 The molar ratio of O and citric acid is 1:1:2, and 16.38g Cu(NO 3 ) 2 ·3H2 O, 27.12g Cr(NO 3 ) 3 9H 2 O and 28.50 g of citric acid were dissolved in 200 mL of deionized water, and the mixed solution was stirred in a water bath at 90 °C until it was in a gel state, then dried at 120 °C for 8 hours, and finally calcined at 600 °C for 4 h in an air atmosphere. Among them, Cr and part of Cu undergo a solid-state reaction to form CuCr 2 o 4 , the remaining Cu forms CuO, giving CuO and CuCr 2 o 4 Mixture, namely CuO-CuCr 2 o 4 powder.
[0019] (2) According to ZrO 2 in ZrO 2 / CuO-CuCr 2 o 4 The mass percent composition in powder is 5%, and 1.08g ZrO(NO 3 ) 2 2H 2 O was dissolved in 20 mL deionized water, and then ZrO(NO 3 ) 2 Add 9.5g CuO-CuCr to the aqueous solution 2 o 4 In the powder, immerse for 5 hours, then dry it in a water bath at 90°C, bake at 120°C for 8 hours, and finally bake at 400°C...
Embodiment 2
[0023] (1) According to Ni(NO 3 ) 2 ·6H 2 O, Cr(NO 3 ) 3 9H 2 The molar ratio of O and citric acid is 1:1:2, and 20.38g Ni(NO 3 ) 2 ·6H 2 O, 28.04g Cr(NO 3 ) 3 9H 2 O and 29.45g of citric acid were dissolved in 200mL of deionized water, and the mixed solution was stirred in a water bath at 90°C until it was in a gel state, then dried at 120°C for 8 hours, and finally calcined at 600°C for 4 hours in an air atmosphere. Among them, Cr and part of Ni undergo a solid-state reaction to form NiCr 2 o 4 , the remaining Ni forms NiO to give NiO and NiCr 2 o 4 Mixture, namely NiO-NiCr 2 o 4 powder.
[0024] (2) According to ZrO 2 in ZrO 2 / NiO-NiCr 2 o 4 The mass percent composition in powder is 5%, and 1.08g ZrO(NO 3 ) 2 2H 2 O was dissolved in 20 mL deionized water, and then ZrO(NO 3 ) 2 Add 9.5g NiO-NiCr to the aqueous solution 2 o 4 In the powder, immerse for 5 hours, then dry it in a water bath at 90°C, bake at 120°C for 8 hours, and finally bake at 400°...
Embodiment 3
[0028] (1) According to Zn(NO 3 ) 2 ·6H 2 O, Cr(NO 3 ) 3 9H 2 The molar ratio of O and citric acid is 1:1:2, and 19.91g Zn(NO 3 ) 2 ·6H 2 O, 26.79g Cr(NO 3 ) 3 9H 2 O and 28.13g of citric acid were dissolved in 200mL of deionized water, and the mixed solution was stirred in a water bath at 90°C until it was in a gel state, then dried at 120°C for 8 hours, and finally calcined at 600°C for 4 hours in an air atmosphere. Among them, Cr and part of Zn undergo a solid-state reaction to form ZnCr 2 o 4 , the remaining Zn forms ZnO, resulting in ZnO and ZnCr 2 o 4 Mixture, namely ZnO-ZnCr 2 o 4 powder.
[0029] (2) According to ZrO 2 in ZrO 2 / ZnO-ZnCr 2 o 4 The mass percent composition in powder is 5%, and 1.08g ZrO(NO 3 ) 2 2H 2 O was dissolved in 20 mL deionized water, and then ZrO(NO 3 ) 2 Add 9.5g ZnO-ZnCr to the aqueous solution 2 o 4 In the powder, immerse for 5 hours, then dry it in a water bath at 90°C, bake at 120°C for 8 hours, and finally bake a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com