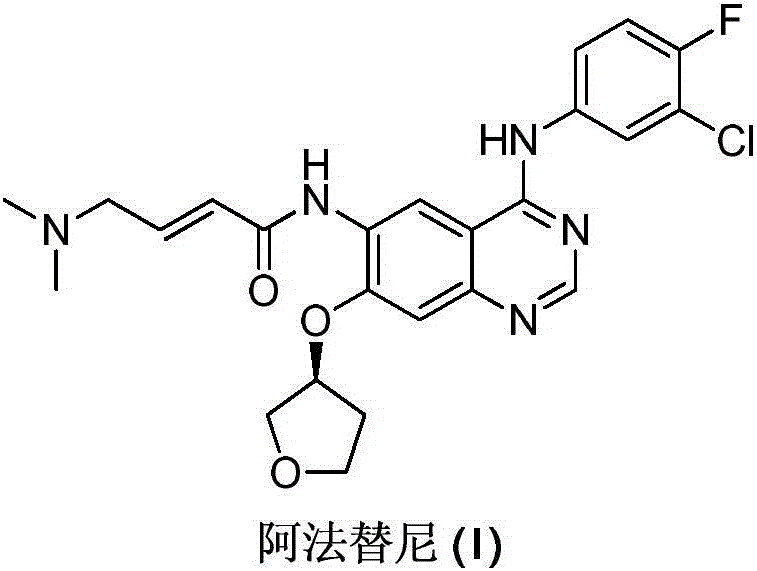
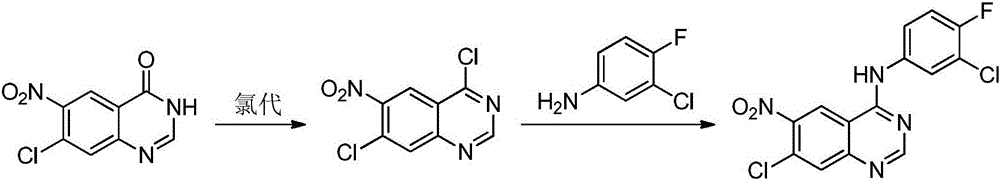
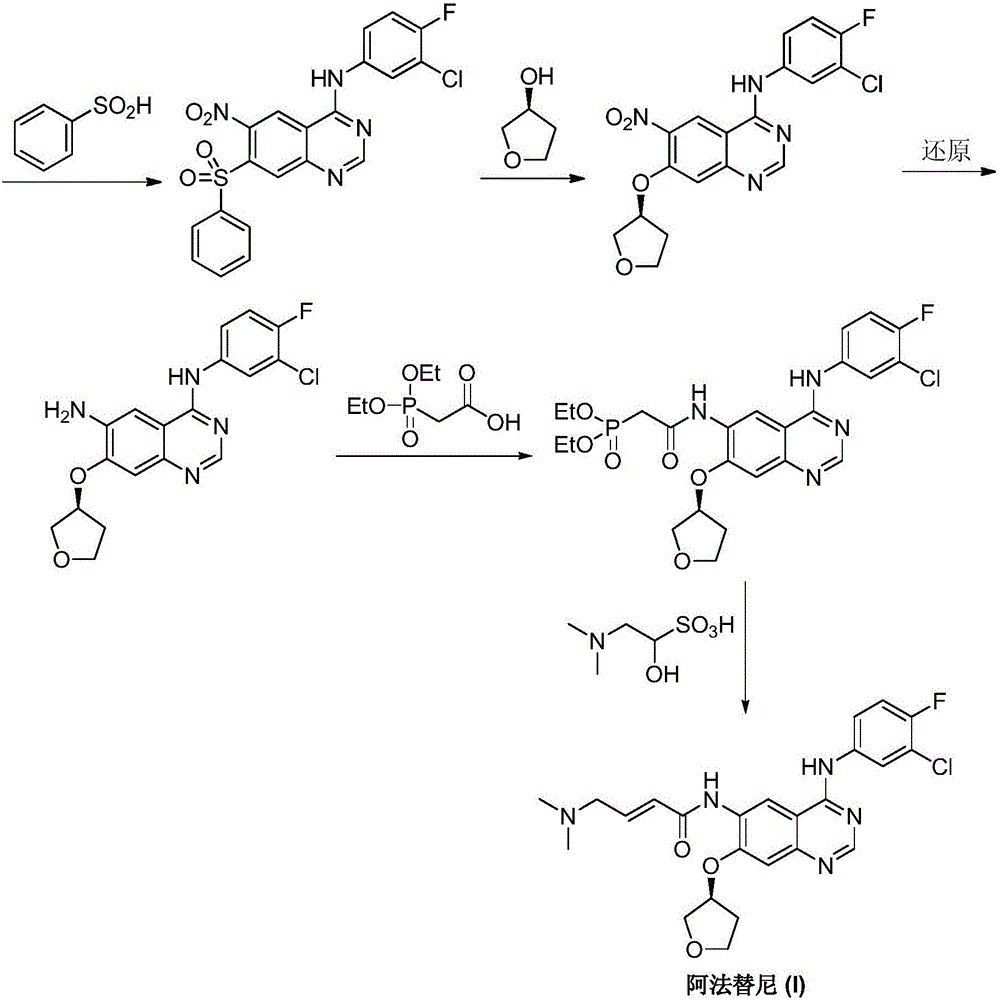
Preparing method of Afatinib
A technology of afatinib and nitro, which is applied in the field of preparation of afatinib, can solve the problems of unfavorable scale-up production and industrial operation, unfavorable industrial production promotion, and affecting the quality of intermediate products, so as to meet the requirements of industrial scale-up production requirements, environmental friendliness, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A) Etherification reaction, dissolve 10.0g (0.113mol) of (S)-3-hydroxytetrahydrofuran in 100mL of tetrahydrofuran, add 2.85g (0.124mol) of sodium metal under nitrogen protection, and stir at 25°C for 2h to obtain (S) )-3-hydroxytetrahydrofuran sodium salt solution in tetrahydrofuran, 21.6g (0.10mol) of 6-nitro-7-fluoro-3,4-dihydroquinazolin-4-one was dissolved in 60mL of tetrahydrofuran, at 30 ℃ Add dropwise to the tetrahydrofuran solution of (S)-3-hydroxytetrahydrofuran sodium salt in a stirring state, reflux at 70°C for 10 hours until the reaction is complete, cool to room temperature, condense to dryness by rotary evaporation under reduced pressure, add 1L ethyl acetate Extract with 1L of water, separate the organic phase, dry over anhydrous sodium sulfate, concentrate to dryness by rotary evaporation under reduced pressure, recrystallize from ethanol, and dry in vacuum at 60°C for 12h to obtain off-white to light yellow powdery solid, that is, to obtain 6-nitro -7-[...
Embodiment 2
[0051] A) Etherification reaction, dissolve 8.8g (0.10mol) of (S)-3-hydroxytetrahydrofuran in 100mL of methyl tert-butyl ether, add 4.0g (0.10mol) of 60% sodium hydride under nitrogen protection, at 25°C Under stirring for 2h, (S)-3-hydroxytetrahydrofuran sodium salt in methyl tert-butyl ether solution, 21.6g (0.10mol) 6-nitro-7-fluoro-3,4-dihydroquinazoline- Dissolve 4-ketone in 60 mL of methyl tert-butyl ether, add dropwise at 30°C to the stirred methyl tert-butyl ether solution of (S)-3-hydroxytetrahydrofuran sodium salt, and react at 40°C for 4h to Complete reaction, cooled to room temperature, concentrated to dryness by rotary evaporation under reduced pressure, added 1L ethyl acetate and 1L water for extraction, separated the organic phase, dried over anhydrous sodium sulfate, concentrated to dryness by rotary evaporation under reduced pressure, recrystallized from ethanol, 60°C Dry in vacuo for 12 hours to obtain off-white to light yellow powdery solid, that is, to obta...
Embodiment 3
[0057] A) Etherification reaction, dissolve 11.5g (0.13mol) of (S)-3-hydroxytetrahydrofuran in 100mL of N,N-dimethylformamide, add 8.6g (0.16mol) sodium methylate under nitrogen protection, 25 Stirring at ℃ for 2h to obtain (S)-3-hydroxytetrahydrofuran sodium salt in N,N-dimethylformamide solution, 21.6g (0.10mol) 6-nitro-7-fluoro-3,4-dihydro Quinazolin-4-one was dissolved in 60 mL of N,N-dimethylformamide, and was added dropwise to the N,N-dimethylformamide of (S)-3-hydroxytetrahydrofuran sodium salt in a stirring state at 30°C. Formamide solution, react at 60°C for 24 hours until the reaction is complete, cool to room temperature, concentrate to dryness by rotary evaporation under reduced pressure, add 1L ethyl acetate and 1L water for extraction, separate the organic phases, dry over anhydrous sodium sulfate, and spin under reduced pressure Evaporate and concentrate to dryness, recrystallize from ethanol, and dry in vacuo at 60°C for 12 hours to obtain off-white to light ye...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com