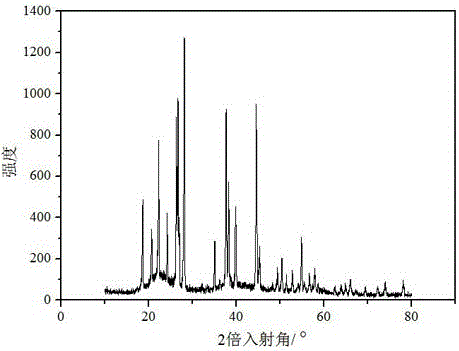
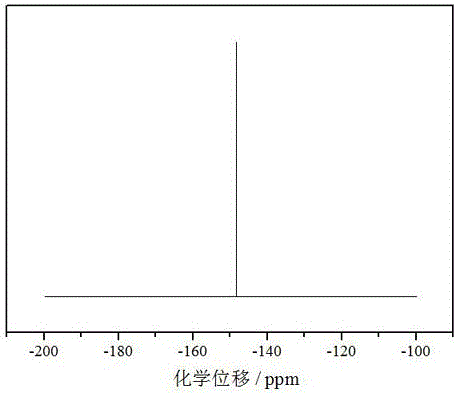
Preparation method for lithium tetrafluoroborate
A technology of lithium tetrafluoroborate and lithium salt, which is applied in the field of preparation, can solve the problems of increased production cost, complicated purification steps, high cost, etc., and achieve the effects of low production cost, high product purity and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0014] The present invention is a kind of preparation method of lithium tetrafluoroborate, and its steps are:
[0015] (1) Lithium salts containing weak acid radicals, BF 3 Compounds of the class of lithium, boron, and fluorine in a molar ratio of 1:1:4 to 1:2:5 are uniformly mixed in an aprotic, nonpolar or less aprotic polar solvent; wherein the solvent and the lithium element The molar ratio is 3:1~6:1;
[0016] (2) In an air atmosphere, reflux reaction at a temperature of 0°C to 70°C for 1 hour to 24 hours, then carry out solid-liquid separation, and then evaporate the solvent in the obtained liquid at 40°C to 120°C, and dry at 30°C Dry the obtained solid at ~130°C for 5-12 hours to obtain LiBF 4 crude product;
[0017] (3) The resulting solid LiBF 4 The crude product is mixed with the aprotic polar solvent at a mass ratio of 1:1 to 1:10, and after reflux at a temperature of 60°C to 100°C for 1 hour to 12 hours, the solid insoluble matter is separated, and the filtrate...
Embodiment 1
[0023] Add 10 g of lithium carbonate and 100 mL of anhydrous diethyl ether which have been pre-dried at 120 °C for 4 hours in a reactor equipped with a stirrer and stir evenly, then add 24.6 mL of BF 3 ·O(C 2 h 5 ) 2 (BF 3 The concentration is 45% ~ 47%) solution. After reflux reaction at 70°C for 6 hours, filter and separate, then evaporate the solvent in the obtained liquid at 65°C, and dry the obtained solid at 80°C for 6 hours to obtain LiBF 4 crude product. The crude product was dissolved in 100 mL of dimethyl carbonate, stirred and refluxed at 90°C for 3 hours. After the solution was filtered, the solvent was evaporated to dryness at 70° C. and 0.085 Mpa (vacuum degree). Then the obtained solid was washed once with 20 mL of anhydrous ether, and finally dried at 110 °C for 6 hours to obtain LiBF 4product. The product yield is 96.5%. LiBF measured by titration 4 The content of B(III) in the product is 11.48% (mass percentage), close to the theoretical value (11.5...
Embodiment 2
[0025] Into a reactor equipped with a stirrer was added 9 g of Li 2 CO 3 and 100 mL of pretreated fresh anhydrous acetonitrile, into which BF was slowly introduced under stirring conditions 3 Gas, heated to 70°C, refluxed for 14 hours, filtered and separated, then evaporated to dryness of the solvent in the obtained liquid at 95°C, and dried the obtained solid at 130°C for 5 hours to obtain a white solid, which was detected as LiBF 4 crude product. The crude product was dissolved in 100 mL propionitrile, stirred and refluxed at 70°C for 3 hours. After the solution was filtered, all the solvent was evaporated at 80°C and 0.08 MPa (vacuum degree) to obtain a solid. Wash with 20 mL of anhydrous diethyl ether twice under the action of suction filtration. Dry the washed product at 120°C for 8 hours to obtain LiBF 4 product. After repeating the above purification process once, LiBF was measured by titration 4 The content of B(III) in the product is 11.50% (mass percentage), c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com