Conjugated dialkynediol compounds and application thereof
A technology of conjugated diacetylenic diols and compounds, applied in organic chemistry, drug combination, nervous system diseases, etc., can solve problems such as failure to protect nerve cells, PC12 cell damage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
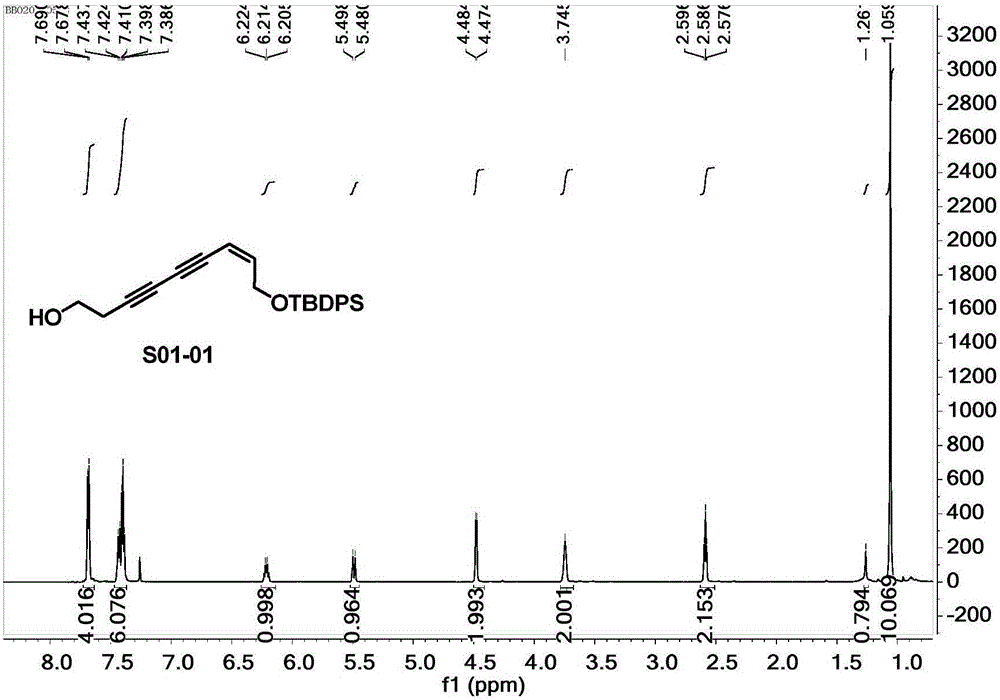
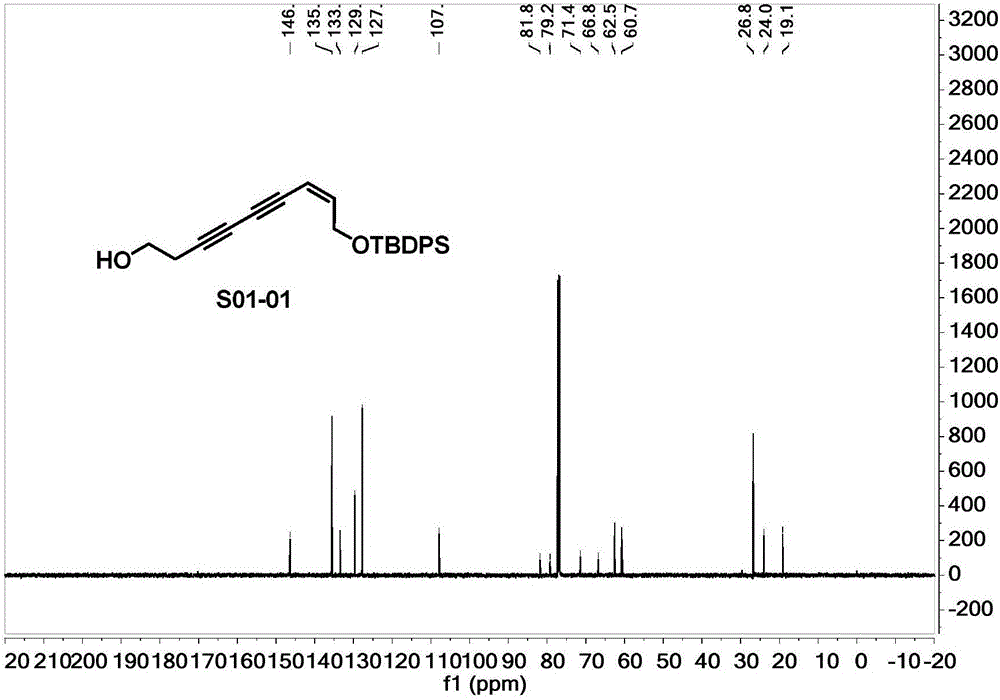
[0038] (Z)-(tert-butyldiphenylsilyl)oxy)-7-nonen-3,5-diyn-1-ol (S01-02), the structure is as follows:
[0039]
[0040] The specific preparation method is:
[0041] 1.1 Take a 10mL round bottom flask, dissolve 12.3μL (0.16mmol) of 3-butyn-1-ol and 5.9mg (0.03mmol) of cuprous iodide in 0.6mL of piperidine under the protection of argon, and put it in an ice-water bath Cool to 0°C, slowly drop (Z)-((5-bromo-2-penten-4-yne-1-oxyl)(tert-butyl)diphenylsilane 50mg (0.13mmol) dropwise at this temperature Dissolve in 0.2mL of piperidine to form a solution, after addition, slowly warm up to room temperature, react at room temperature for 4h, add 0.5mL of saturated ammonium chloride solution to terminate the reaction, and then extract with ethyl acetate (0.3mL×2) , combine the organic phases, wash with saturated NaCl solution, dry over anhydrous sodium sulfate, filter, evaporate the solvent under reduced pressure, use V (petroleum ether): V (ethyl acetate) = 2:1 as eluent, silica gel...
Embodiment 2
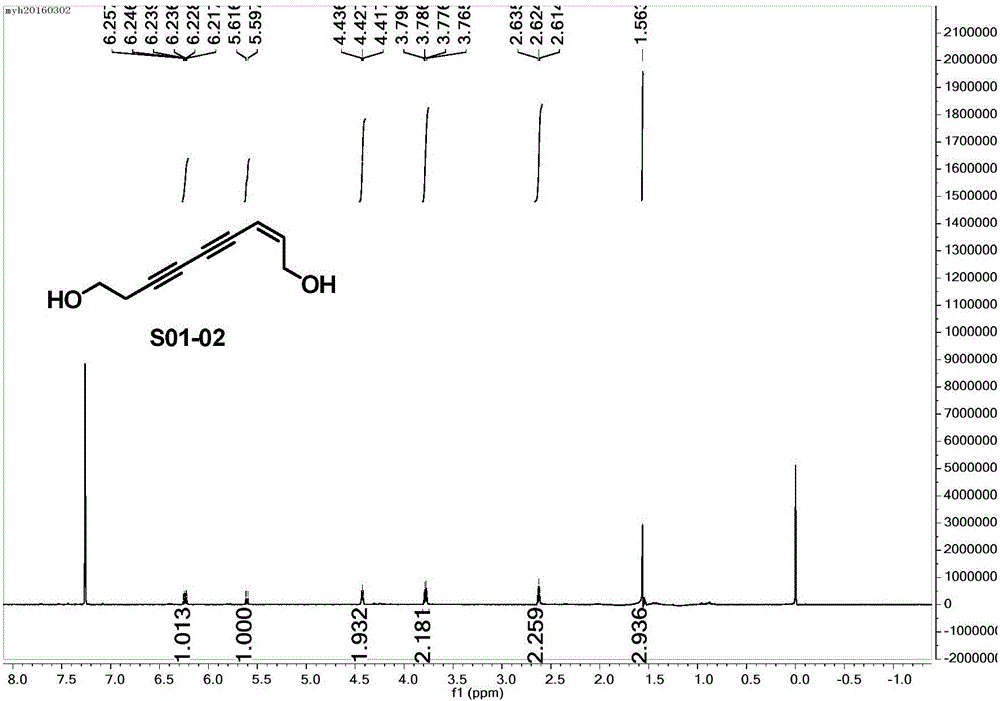
[0052] Example 2 (2Z,8E)-2,8-decadiene-4,6-diyne-1,10-diol (S02-02), the structure is as follows:
[0053]
[0054] The specific preparation method is:
[0055] 2.1 Replace the 3-butyn-1-ol in Step 1.1 of Example 1 with (E)-2-penten-4-yn-1-ol, and the remaining raw material reagents and preparation methods are the same as in Example 1.1 to obtain Product (2E,8Z)-10-((tert-butyldiphenyl)oxy)-2,8-decadien-4,6-diyn-1-ol (S02-01). Colorless oily liquid, yield 36%.
[0056] In order to further prove that (2E,8Z)-10-((tert-butyldiphenyl)oxy)-2,8-decadiene-4,6-diyn-1-ol (S02-01 ), carried out nuclear magnetic resonance detection to it, obtained proton nuclear magnetic resonance spectrum and carbon spectrum, see respectively Figure 5 with Image 6 , the specific test results are as follows:
[0057] 1 H NMR (600MHz, CDCl 3 )δ:7.69-7.68(m,4H),7.44-7.39(m,6H),6.41-6.37(m,1H),6.24-6.21(m,1H),5.84(d,J=15.6Hz,1H) ,5.55(d,J=11.4Hz,1H),4.48(d,J=6.0Hz,2H),4.25(d,J=4.2Hz,2H),1.26(s...
Embodiment 3
[0066] Example 3. (Z)-7-(4-(hydroxymethyl)phenyl)-2-heptene-4,6-diyn-1-ol (S03-02), the structure is as follows:
[0067]
[0068] The specific preparation method is:
[0069] 3.1 Replace 3-butyn-1-alcohol in Step 1.1 of Example 1 with 4-ethynyl benzyl alcohol, and the remaining raw material reagents and preparation methods are the same as in Example 1.1 to obtain (Z)-(4-(7- ((tert-butyldiphenyl)oxy)-5-heptene-1,3-diyne-benzyl alcohol (S03-01), light yellow oily liquid, yield 70%.
[0070] In order to further prove (Z)-(4-(7-((tert-butyldiphenyl)oxy)-5-heptene-1,3-diyne-benzyl alcohol (S03-01) in this example, Carried out nuclear magnetic resonance detection to it, obtained proton nuclear magnetic resonance spectrum and carbon spectrum, see respectively Figure 9 with Figure 10 , the specific test results are as follows:
[0071] 1 H NMR (600MHz, CDCl 3 )δ: 7.69-7.68(m, J=7.8Hz, 4H), 7.47(d, J=7.8Hz, 2H), 7.44-7.39(m, 6H), 7.33(d, J=7.8Hz, 2H), 6.27-6.23(m,1H),5.59(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com