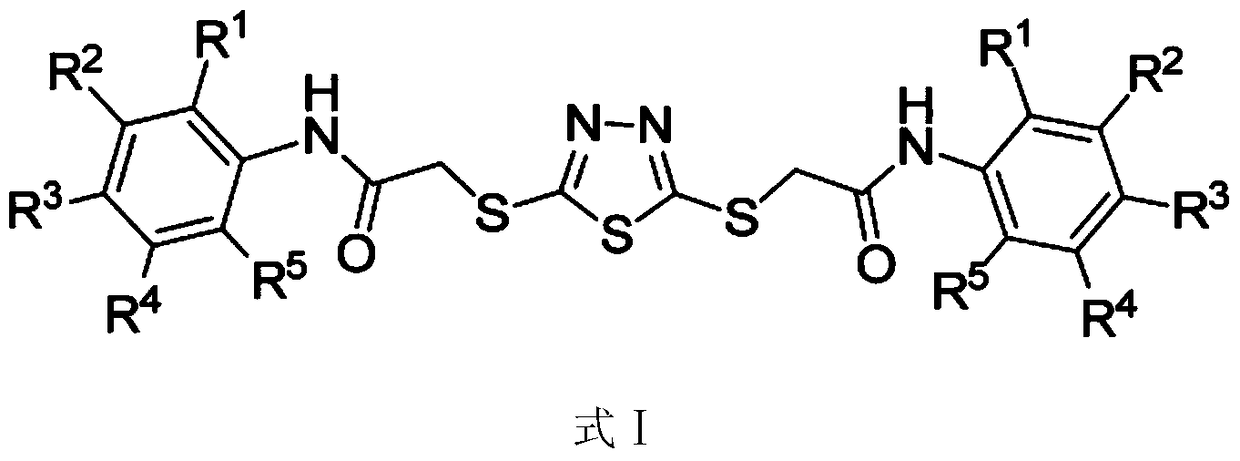
Application of 2,5-bis(substituted carbamoylmethylthio)-1,3,4-thiadiazoles in inhibiting the growth of cyanobacteria
A carbamoylmethylthio and thiadiazole technology, applied in the application of inhibiting the growth of cyanobacteria, in the field of 2,5-di-1,3,4-thiadiazole compounds, which can solve the problem that cyanobacteria cannot grow or even death, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
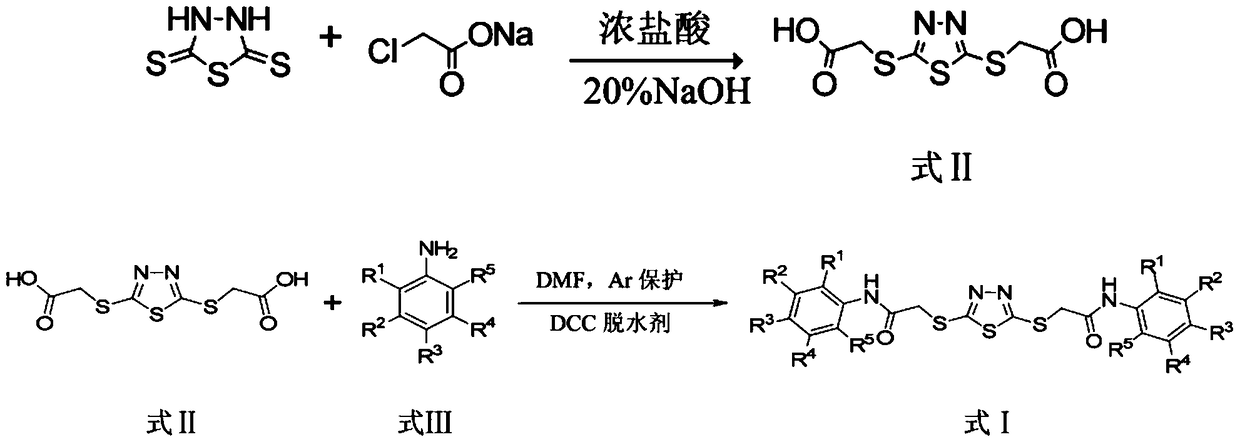
[0021] The preparation method of the compound shown in formula I comprises the following steps: (1) reacting 2,5-dimercapto-1,3,4-thiadiazole and sodium chloroacetate under alkaline conditions, then acidifying to obtain the reaction intermediate (compound shown in formula II); (2) intermediates and anilines (compound shown in formula III, R 1 , R 2 , R 3 , R 4 , R 5 Same as above) react under the protection of dehydrating agent DCC and argon (Ar), and the corresponding target product can be obtained after post-treatment. The reaction formula of step (1) and (2) is as follows:
[0022]
[0023] The reaction in step (2) above uses DCC as a dehydrating agent, and the by-product N,N'-dicyclohexylurea can be removed after the reaction solution is poured into water, and the target product can be precipitated. Suction filtration and ethanol recrystallization gave pure product.
Embodiment 1
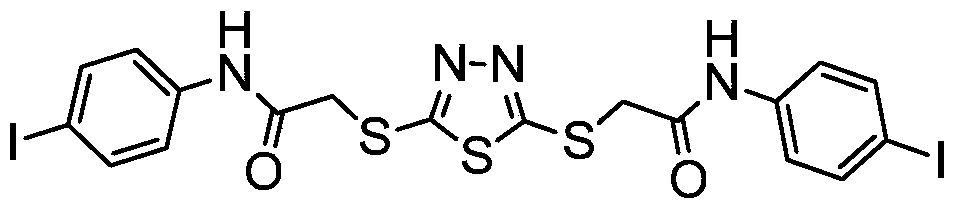
[0025] Preparation of compound I-12,5-bis(p-iodophenylcarbamoylmethylthio)-1,3,4-thiadiazole
[0026]
[0027] (1) Dissolve 20mmol of 2,5-dimercapto-1,3,4-thiadiazole into 8.0mL of 20% sodium hydroxide solution, add 37.8mL of 30% sodium chloroacetate solution dropwise under stirring, and control the pH The value is between 10 and 11, and the reaction is 6h. Concentrated hydrochloric acid was acidified to pH = 2, and a large amount of white precipitate was obtained. Suction filtration, washing with water, drying, recrystallization with ethanol, drying again to obtain pure compound II.
[0028] (2) Add 1.5 mmol of compound II and 3.3 mmol of p-iodoaniline into 3.0 mL of anhydrous DMF, protect with argon, and cool to 0-5° C. in an ice-water bath. Dissolve 0.31g of DCC in 1.0mL of anhydrous DMF, then add the DCC solution to the above mixture, and complete the addition within 3 minutes. Stir at room temperature for 2h and let stand overnight. After filtration, the filtrate w...
Embodiment 2
[0136] The 2,5-bis(substituted carbamoylmethylthio)-1,3,4-thiadiazole compounds prepared in Example 1 were subjected to cyanobacterial fructose-1,6- / sedoheptulose-1,7 - Bisphosphatase inhibition test. Cyanobacterial fructose-1,6- / sedum heptulose-1,7-bisphosphatase hydrolyzes fructose-1,6-bisphosphate into fructose-6-phosphate and inorganic phosphate. Therefore, the activity of cyanobacterial fructose-1,6- / sedum heptulose-1,7-bisphosphatase can be determined by measuring the increase of inorganic phosphate, and then reflect the above-mentioned 2,5-bis(substituted carbamoyl methyl Sulfuryl)-1,3,4-thiadiazole compounds as inhibitors of inhibitory efficiency. The specific operation is as follows:
[0137] (1) Weigh the compound in advance, dissolve it in DMSO, and prepare the above-mentioned 2,5-di(substituted carbamoylmethylthio) with different concentrations of 0, 0.01, 0.03, 0.1, 0.3, 1, 3, 10, 30mM, etc. -1,3,4-thiadiazole compounds.
[0138](2) The reaction system for mea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com