Catalyst for improving syngas converted product selectivity and preparation method and application thereof
A conversion product and catalyst technology, applied in the preparation of organic compounds, preparation of liquid hydrocarbon mixtures, metal/metal oxide/metal hydroxide catalysts, etc. Alcohol selectivity is not high
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
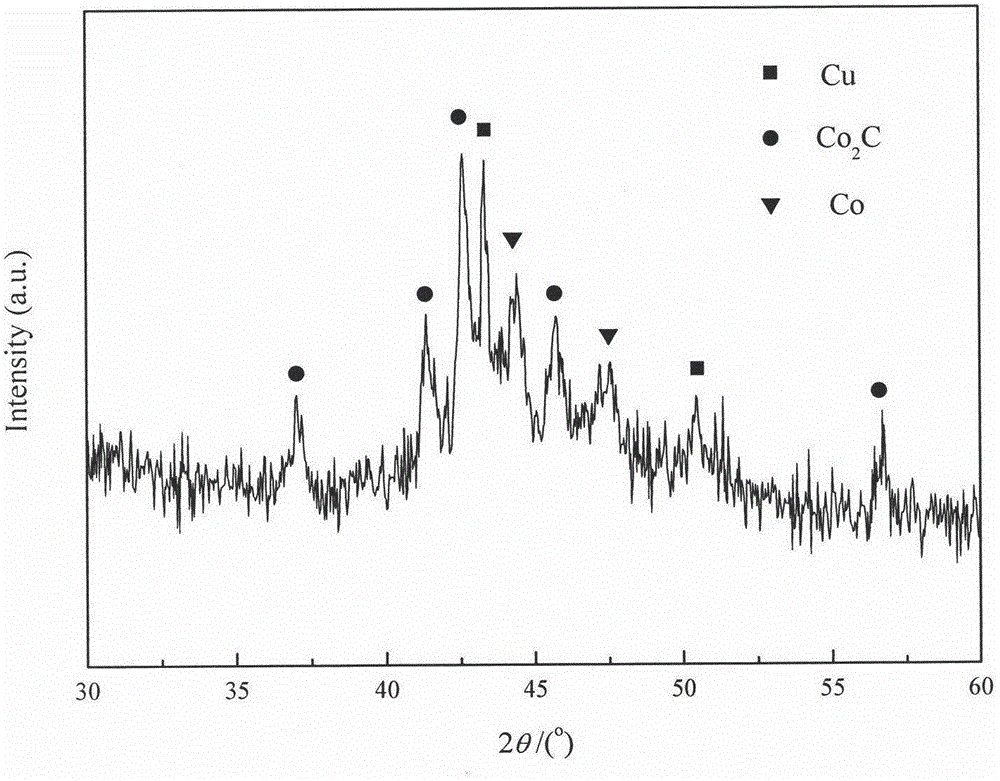
[0025] Take by weighing 3.704g cobalt nitrate (Co(NO 3 ) 2 ·6H 2 O) and 0.755g copper nitrate (Cu(NO 3 )·3H 2 0), put into the 50mL beaker that fills 5mL aqueous solution, stir until cobalt nitrate and copper nitrate dissolve completely, add the manganese nitrate solution miscibility of 0.324g 50%. Take 5g of AC carrier, immerse the carrier in the impregnation solution containing the active component, let it stand for 1h, make the active component and co-active component loaded on the carrier by capillary action, and then dry it in the air at 60°C for 12h, the obtained sample Calcined at 350° C. for 4 h in an Ar atmosphere, and then cooled to room temperature to obtain a black sample. The above catalyst is named as A, and the catalyst composition is listed in Table 1. The physical properties of the catalysts are listed in Table 2.
[0026] Take 4mL of the above-mentioned catalyst for reduction treatment, the conditions and steps adopted are as follows: pure hydrogen with...
Embodiment 2
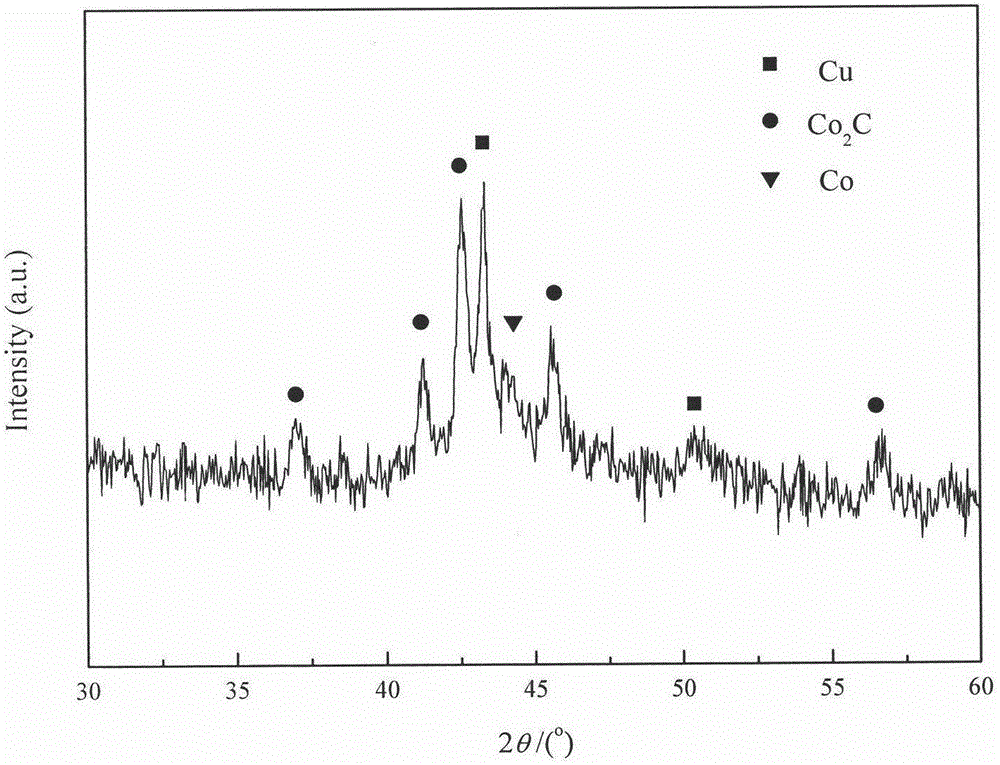
[0028] Take by weighing 3.704g cobalt nitrate (Co(NO 3 ) 2 ·6H 2 O) and 0.755g copper nitrate (Cu(NO 3 )·3H 2 0), put into the 50mL beaker that fills 5mL aqueous solution, stir until cobalt nitrate and copper nitrate dissolve completely, add the manganese nitrate solution miscibility of 1.302g 50%. Take 5g of AC carrier, immerse the carrier in the impregnating liquid containing the active component, let it stand for 1 hour, make the active component and co-active component loaded on the carrier through capillary action, and then dry it in the air at 60°C for 12h, The obtained sample was calcined at a constant temperature of 350° C. for 4 h in an Ar atmosphere, and then cooled to room temperature to obtain a black sample. The above catalyst is named B, and the catalyst composition is listed in Table 1. The physical properties of the catalysts are listed in Table 2.
[0029] Take 4mL of the above-mentioned catalyst for reduction treatment, the conditions and steps adopted ...
Embodiment 3
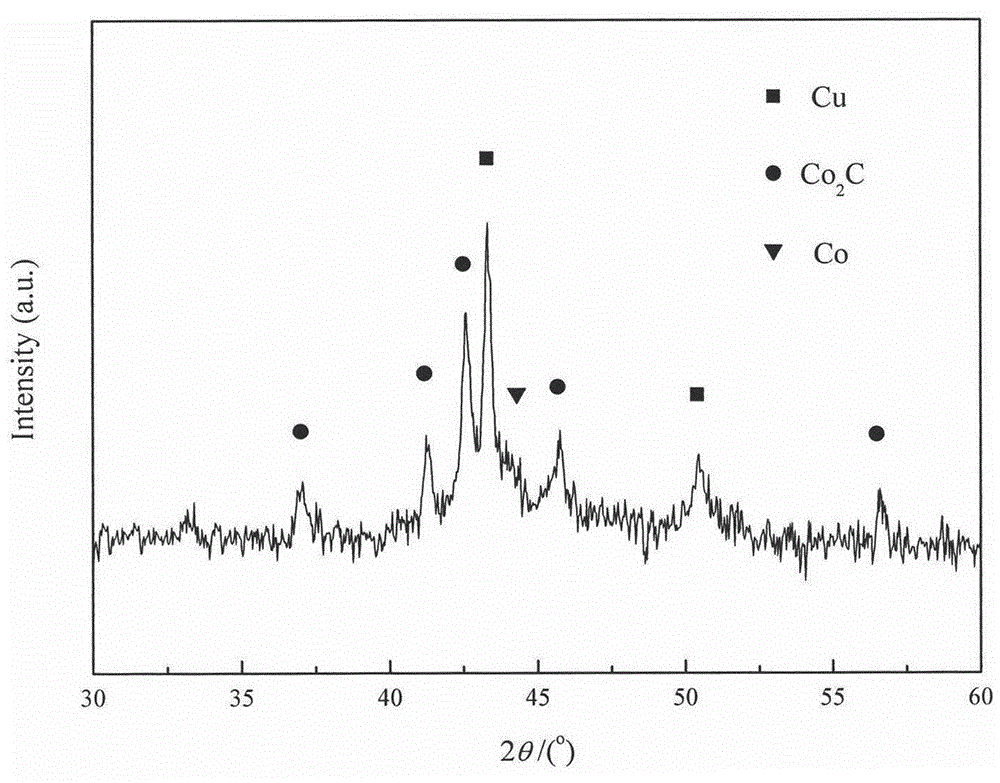
[0031] Take by weighing 3.704g cobalt nitrate (Co(NO 3 ) 2 ·6H 2 O) and 1.51g copper nitrate (Cu(NO 3 )·3H 2 0), put into the 50mL beaker that fills 5mL aqueous solution, stir until cobalt nitrate and copper nitrate dissolve completely, add the manganese nitrate solution miscibility of 2.605g 50%. Take 5g of AC carrier, immerse the carrier in the impregnating solution containing the active component, let it stand for 1 hour, make the active component and co-active component loaded on the carrier through capillary action, and then dry it in the air at 60°C for 12 hours, The obtained sample was calcined at a constant temperature of 350° C. for 4 h in an Ar atmosphere, and then cooled to room temperature to obtain a black sample. The above catalyst is named C, and the catalyst composition is listed in Table 1. The physical properties of the catalysts are listed in Table 2.
[0032] Take 4mL of the above-mentioned catalyst for reduction treatment, the conditions and steps ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com