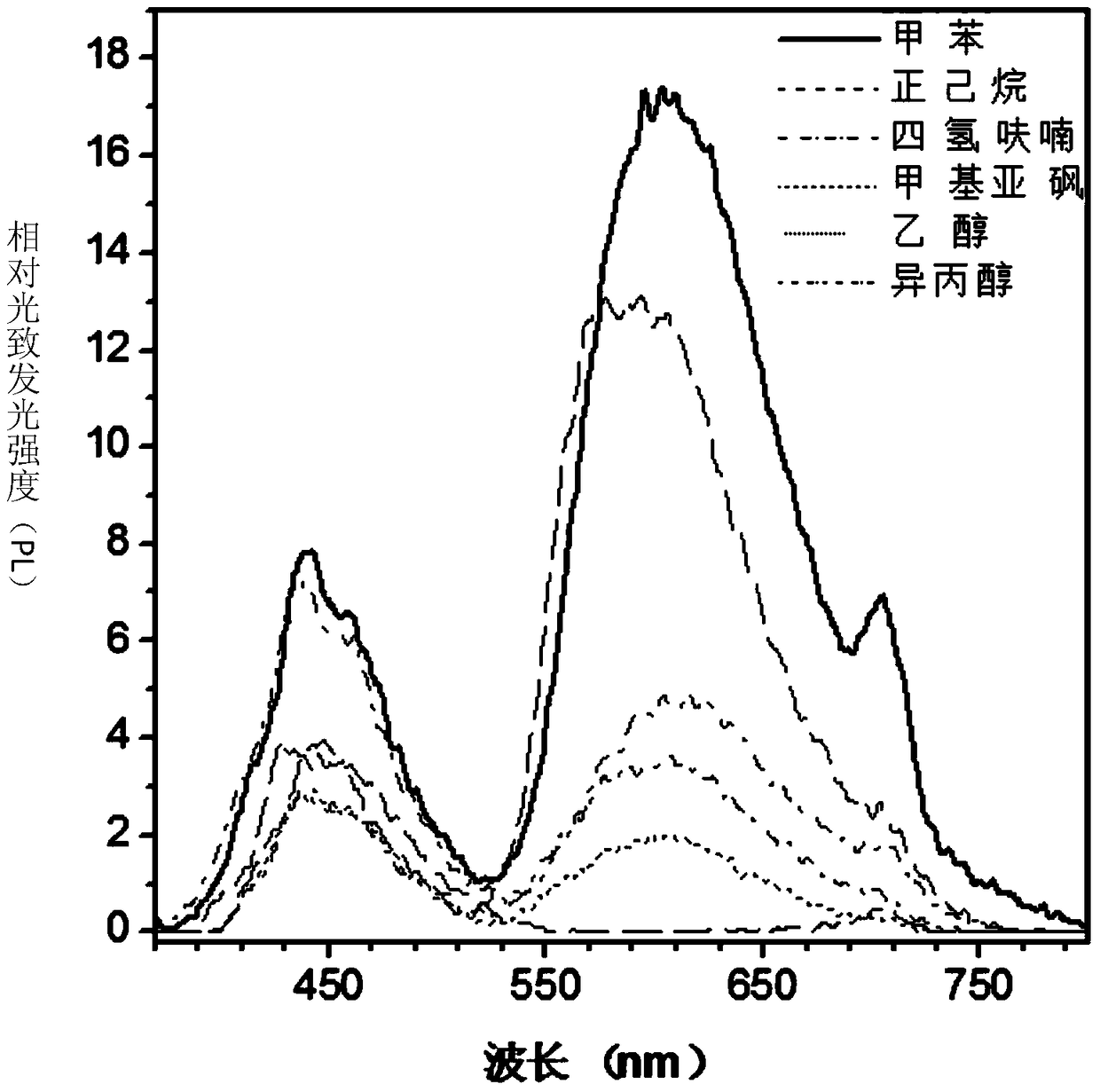
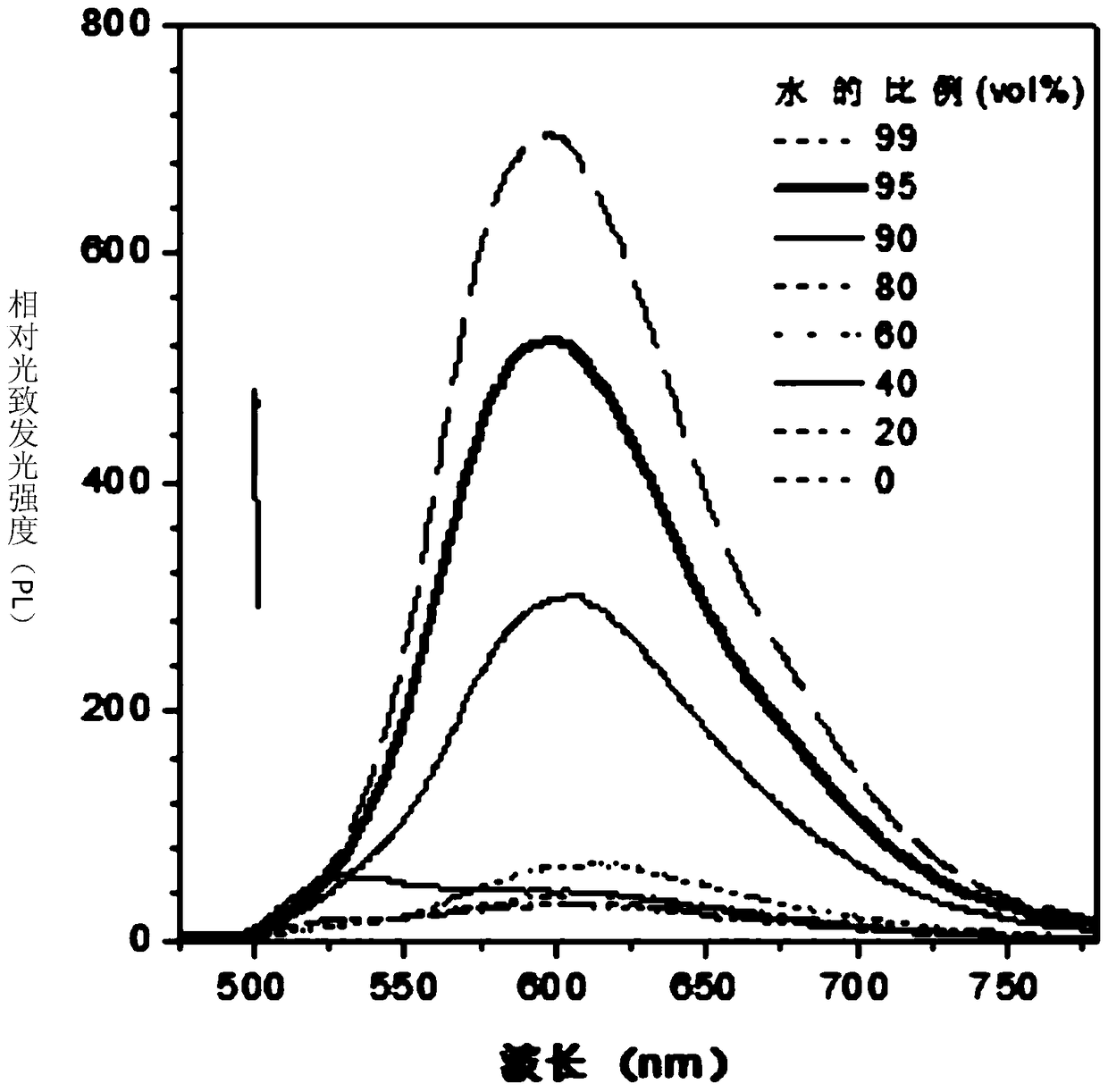
Fluorenyl salicylaldehyde hydrazine derivatives, preparation method and application thereof
A technology of fluorenyl salicylaldehyde hydrazine, which is applied in the field of fluorenyl salicylaldehyde hydrazine derivatives and their preparation, can solve the problems of difference and large fluorescence shift, and achieve increased Stokes shift and electron-withdrawing ability , The effect of preventing self-absorption phenomenon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Embodiment 1: the synthesis (FAS) of fluorenyl salicylaldehyde hydrazine
[0066]
[0067] Reflux 10g of fluorenone and 20ml of hydrazine hydrate (85%) under 100ml for 4h. After cooling, a large number of needle-like solids precipitated. After filtering, recrystallize twice with ethanol to obtain light yellow needle-packed crystals with a purity of 99% and a yield of 95%. %; take 2g of hydrazine hydrate crystals and 2ml of salicylaldehyde in 20ml of ethanol to reflux for 4h, after cooling to obtain light yellow needle-packed crystals, after filtering, wash with 75% ethanol solution to obtain the FAS structure, the yield is 99%. MALDI-TOF(m / z):[M+]calcd.C 20 h 14 N 2 O, 298.34; found, 299.34. Anal Calc. for C 20 h 14 N 2 O: C, 80.52; H, 4.73; N, 9.39; O, 5.36. Found: C, 80.62; H, 4.71; N, 9.19; O, 5.36.
Embodiment 2
[0068] Embodiment 2: Synthesis of FAS derivatives (1)
[0069]
[0070] Get 500mg of hydrazine hydrate crystals and equimolar m-hydroxysalicylaldehyde (or m-bromosalicylaldehyde) derivatives to react in 10ml of ethanol at 60°C for 4h. After cooling, light yellow crystals are obtained. After filtration, use 75% ethanol solution After cleaning, the corresponding FAS-derived structure was obtained by chromatography of the product, and the yield was >90%.
[0071] m-FAS-OH:MALDI-TOF(m / z):[M+]calcd.C 20 h 14 N 2 o 2 ,314.34; found, 315.24.AnalCalc.for C 20 h 14 N 2 o 2 H, 4.49; N, 8.91; O, 10.18. Found: C, 75.32; H, 4.48; N, 8.35; O, 10.09.
[0072] p-FAS-Br:MALDI-TOF(m / z):[M+]calcd.C 20 h 13 BrN 2 O, 377.23; found, 299.34. AnalCalc.for C 20 h 13 BrN 2 O: C, 63.68; H, 3.47; Br, 21.18; N, 7.43; O, 4.24. Found: C, 63.58; H, 3.40;
Embodiment 3
[0073] Example 3: Synthesis of FAS derivatives (2)
[0074]
[0075] Take 500mg of hydrazine hydrate crystals and equimolar N,N-diethylaminosalicylaldehyde in 10ml of ethanol and react at 60°C for 4 hours. After cooling, a red oily substance is obtained. After filtering, wash with 75% ethanol solution to obtain the product layer The corresponding FAS derivative structure was obtained by analysis with a yield of 85%.
[0076] p-FAS-N22: MALDI-TOF(m / z):[M+]calcd.C 24 h 23 N 3 O: 369.18; found, 370.66; AnalCalc.for C 24 h 23 N 3 O: C, 78.02; H, 6.27; N, 11.37; O, 4.33. Found, C, 77.93; H, 6.10; N, 11.25; O, 4.45.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com