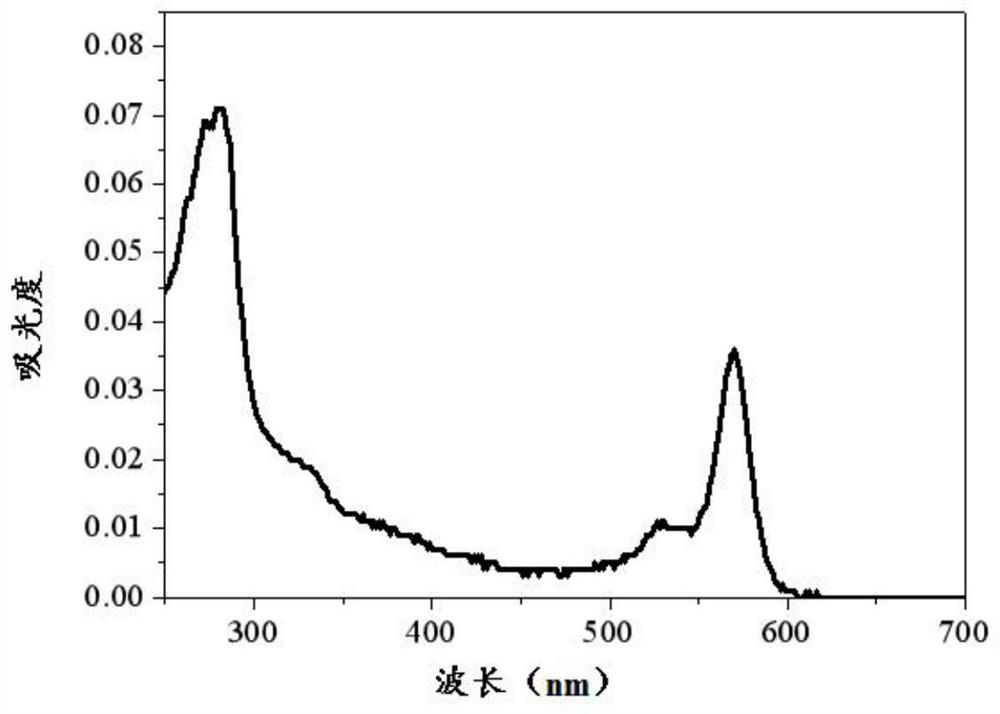
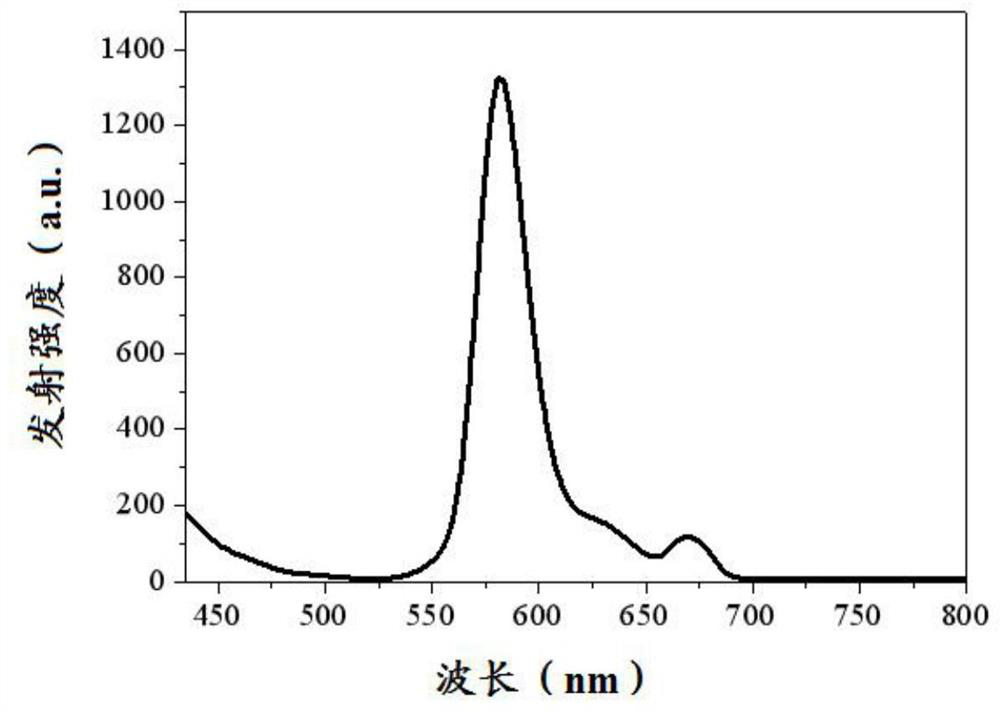
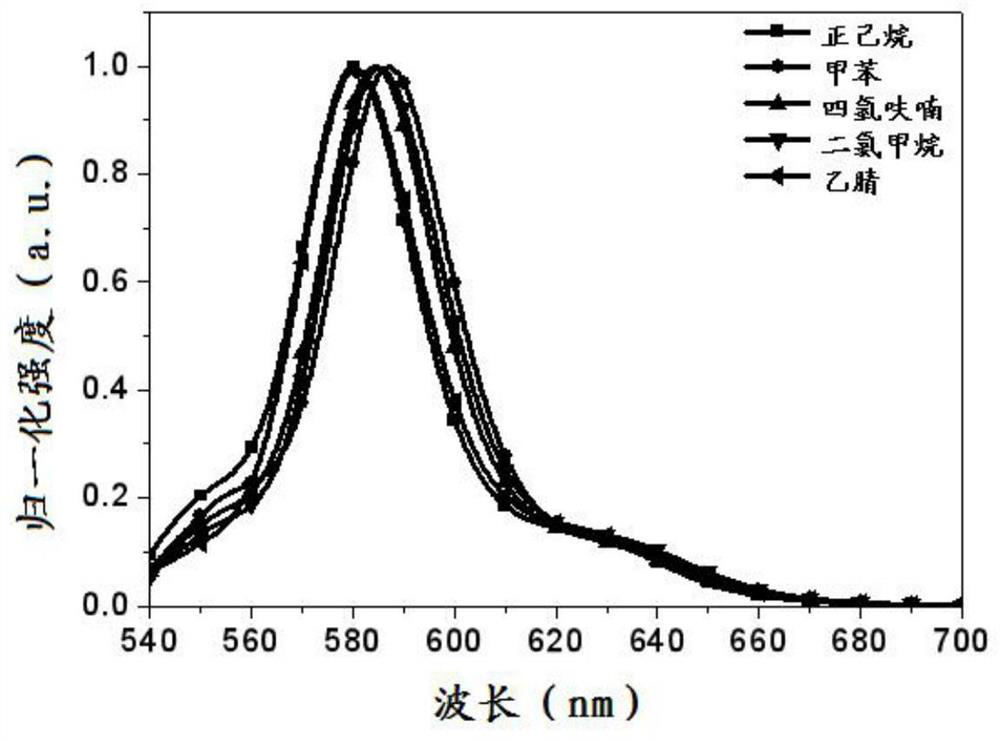
Fluorescent boron dipyrrole fluorescent dye and its preparation method and application
A technology of fluorobodipyrrole and fluorescent dyes, which is applied in the field of fluorescent dyes, can solve the problems of small red shift and limited application, and achieve the effect of improving electron-withdrawing ability and high quantum yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The present invention also provides a method for preparing the fluorobodipyrrole fluorescent dye described in the above technical solution, comprising the following steps:
[0028] mixing pyrrole, p-tolualdehyde and trifluoroacetic acid, and carrying out condensation reaction under a protective atmosphere to obtain the intermediate shown in formula II;
[0029] Under a protective atmosphere, mix the tetrahydrofuran solution of the intermediate represented by formula II and N-bromosuccinimide at -80 to -76°C, and after the N-bromosuccinimide is dissolved, add 2 , 3-dichloro-5,6-dicyano-1,4-benzoquinone in tetrahydrofuran solution, and then carry out bromination reaction at room temperature to obtain the intermediate shown in formula III;
[0030] Mixing the intermediate represented by the formula III with triethylamine, boron trifluoride ethyl ether, and dichloromethane, and performing a fluoroboration reaction to obtain the intermediate represented by the formula IV;
...
Embodiment 1
[0061] (1) Preparation of intermediates shown in formula Ⅱ
[0062] Pyrrole (27.8mL, 400mmol) was mixed with p-tolualdehyde (2.36mL, 20mmol), stirred and reacted under nitrogen atmosphere for 15min, then added trifluoroacetic acid (0.15mL, 2mmol), stirred and reacted for 14h, and then the resulting reaction solution was mixed with Mix 50mL of 0.1M sodium hydroxide solution and 100mL of dichloromethane for the first extraction, wash the obtained organic phase twice with 50mL of water each time, then wash with saturated saline and anhydrous magnesium sulfate in sequence Dry, then carry out vacuum distillation to remove unreacted pyrrole, and carry out the first column chromatography of the obtained residue, the eluent used is petroleum ether, ethyl acetate and triethylamine according to the volume ratio of 8:2:1 mixed The obtained mixed solution was vacuum-dried the obtained fraction containing the target product to obtain the intermediate represented by formula II as a reddish-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com