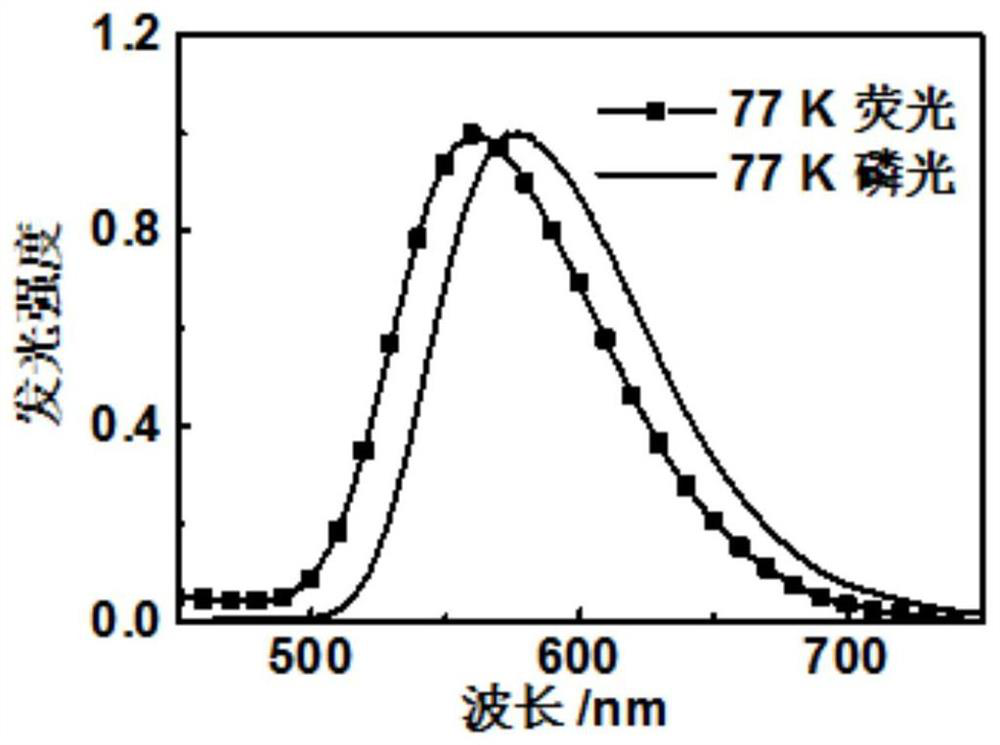
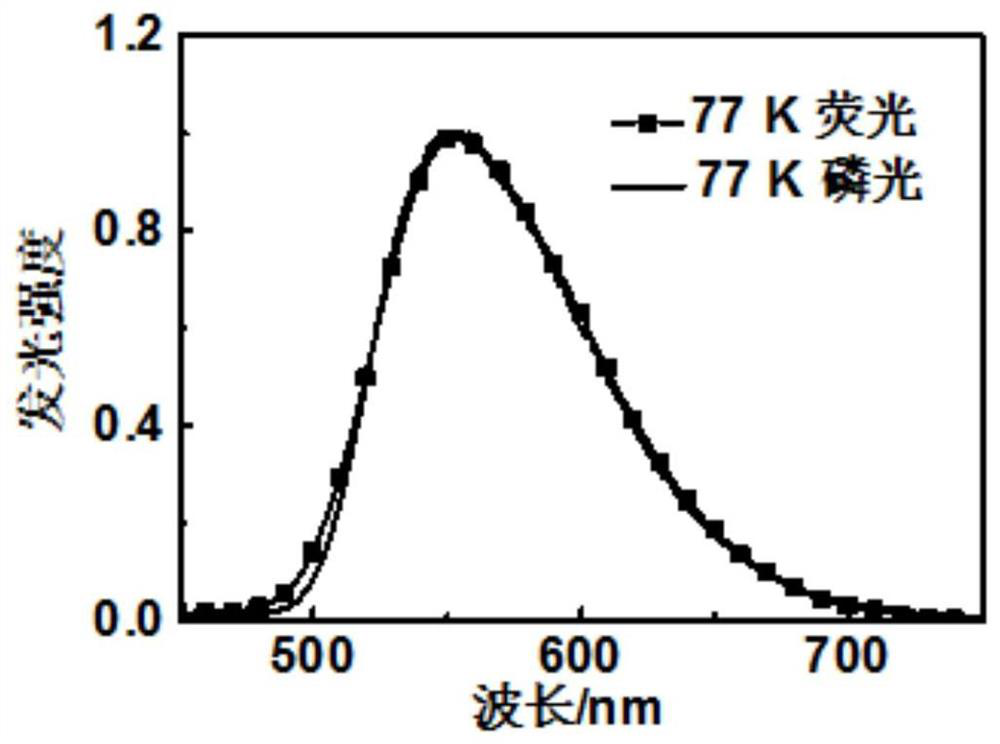
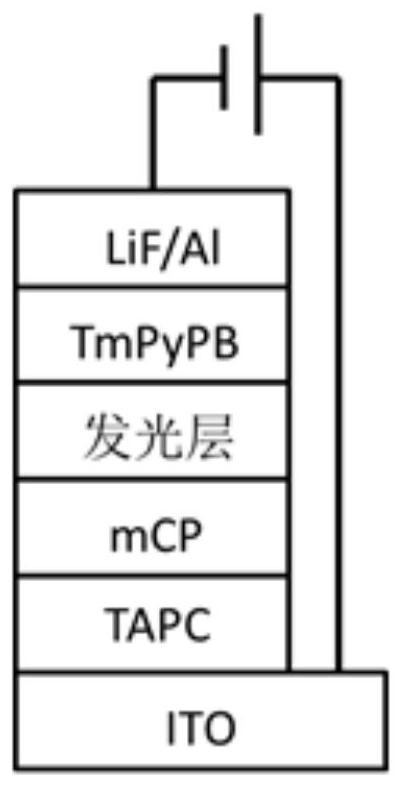
A kind of dithioxanthone derivatives and its preparation method and application
A technology of thioxanthone and its derivatives, which is applied in the field of organic electroluminescence display, can solve the problems that it is not suitable for the preparation of high color rendering index white light OLED devices, and cannot realize light emission at different positions, so as to improve the electron and hole transport performance , high color rendering index, and the effect of widening the conjugate length
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0123] Synthesis of 2,9-dibromothiochromeno[2,3-b]thioxanthene-7,14-dione:
[0124]
[0125] Dissolve 150mg of compound 1 and 180mg of compound sodium hydride in 10ml of ultra-dry N,N-dimethylformamide (DMF), vacuumize and blow with nitrogen. 500 mg of compound 2 was dissolved in 10 ml of DMF, and added to the solution of compound 1, and the reaction mixture was heated to 130° C. for 5 h. 100ml of water was added to the reacted solution, the precipitate was filtered out, and 438mg of product 3 was obtained by column chromatography.
[0126] Mix 200mg of compound 3, 5g of potassium hydroxide, 10ml of water, and 10ml of ethanol in a flask, heat it to 100°C until the reaction solution becomes clear and then cool down to room temperature, add excess hydrochloric acid to precipitate the precipitate, filter the precipitate, and dry it in vacuum Oven drying yielded 210 mg of compound 4.
[0127]200mg of compound 4 was dissolved in 10ml of concentrated sulfuric acid, heated to 60...
Embodiment 2
[0129] Synthesis of 2,9-dibromothiochromeno[2,3-b]thioxanthene-7,14-dione oxidation product: Dissolve 200mg of compound a-1 in 20ml of dichloromethane in an ice-water bath, slowly add 300mg, The reaction solution was moved to room temperature for overnight reaction. The reaction solution was concentrated, and column chromatography obtained 106 mg of the compound represented by the product formula a-2. 1H NMR (400MHz, TFA) δ8.22 (s, 2H), 8.09 (d, J = 1.5Hz, 2H), 7.93 (dd, J = 7.5, 1.5Hz, 2H), 7.75 (d, J = 7.5Hz ,2H).EIMS m / z(%):calcd for C20H8Br2O6S2,567.81; Found:567.81.
[0130]
Embodiment 3
[0132] Synthesis of Triphenylamine-substituted Dithioxanthone Derivatives Comp-1
[0133] Weigh 200mg of 2,9-dibromothiochromeno[2,3-b]thioxanthene-7,14-dione and 650mg of 9-triphenylamine boronic acid into a 250mL two-necked bottle, add 50mg of tetrakistriphenyl Phosphine palladium, 15mL of 2M potassium carbonate aqueous solution and 40mL of toluene solvent, after reflux reaction under nitrogen protection for 5h, the solvent was removed under reduced pressure, extracted with dichloromethane and water, the organic phases were combined, the solvent was evaporated to dryness under reduced pressure, and column chromatography was obtained m-chloroperoxybenzoic acid to dithioxanthone derivatives Comp-1.
[0134]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Maximum brightness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com