Dust mite allergen and application thereof
An allergen and dust mite technology, applied in the field of biomedicine, can solve problems such as complex components, affecting repeatability and safety, and pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1. Discovery of Dust Mite Allergen Translation Elongation Factors
[0031] The gene sequence of the translation elongation factor of D. farinae was obtained by analyzing and comparing the whole genome sequence of D. farinae obtained by cooperation between Shenzhen University and Chinese University of Hong Kong.
Embodiment 2
[0032] Example 2. Molecular cloning of dust mite allergens
[0033] 1. Extraction of total RNA of dust mite
[0034] Pick clean live D. farinae, and use the RNeasy Mini Kit from Qicgen Company to extract total RNA. The operation steps are carried out according to the instructions.
[0035] 2. Der f 35 full-length cDNA clone
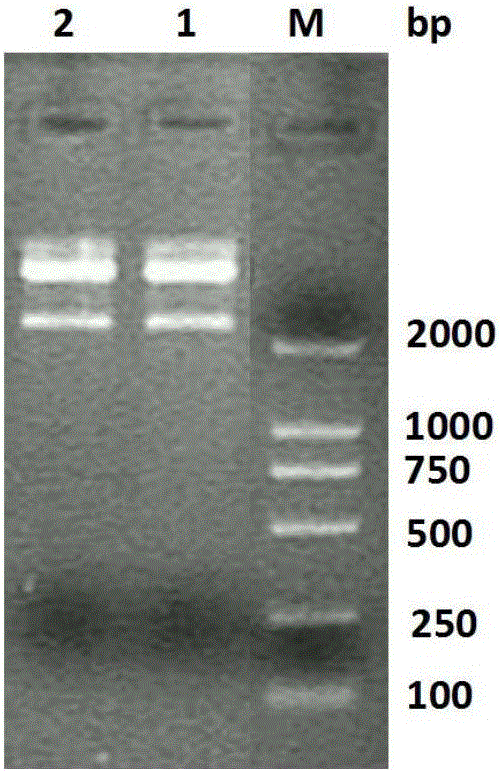
[0036] Using the extracted total RNA as a template, cDNA was reverse-transcribed and PCR amplification was performed. The reaction system is as follows (50 μL): 5 μL of 10×Ex Taq Buffer; 0.25 μL of TaKaRa ExTaq; 4 μL of dNTP Mixture; 2 μL of upstream and downstream primers, 1 μL of cDNA as template; add deionized water to 50 μL. PCR reaction conditions: denaturation at 94°C for 1 min; annealing at 50°C for 1 min; extension at 72°C for 1 min; 35 cycles. PCR products were verified by 1% agarose electrophoresis and photographed.
[0037] 3. Recombinant plasmid construction and enzyme digestion identification
[0038] After the above PCR product was conne...
Embodiment 3
[0043] Example 3: Detection of Allergenicity of Dust Mite Allergens
[0044] 1. Elisa experiment
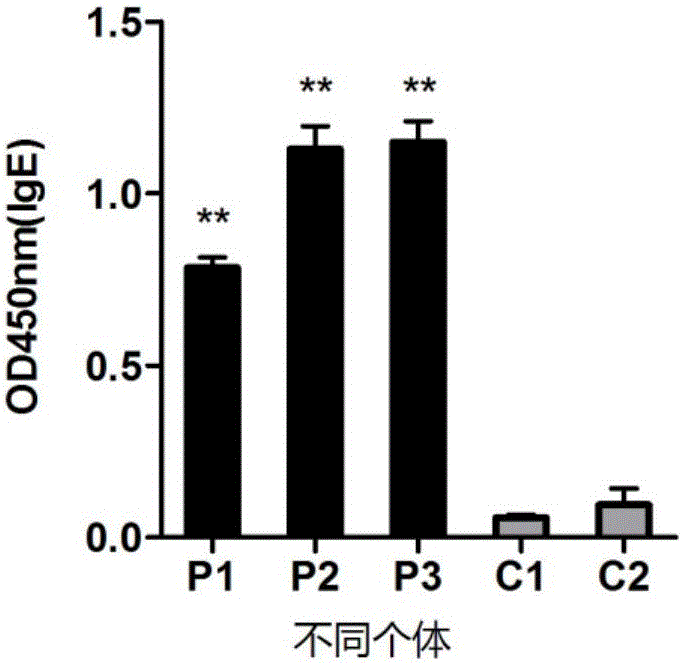
[0045] Dilute the allergen to 10ug / ml with coating solution, coat with 50ul overnight at 4°C, dilute the patient’s serum at 1:5, dilute the secondary antibody at 1:2000, and finally measure the light absorption value of OD450nm.
[0046] The Elisa results of the effect of Der f 35 on serum IgE of dust mite allergic patients are as follows: image 3 as shown, image 3 Among them, P1-P3 are the results of different asthmatic patients, and C1-C2 are the results of different healthy people.
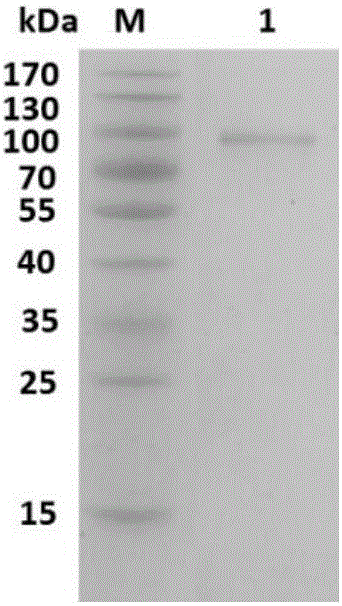
[0047] 2. Western blot experiment
[0048] Western blotting was performed with the serum of patients allergic to dust mite as the primary antibody, and goat anti-human IgE labeled with streptavidin-horseradish peroxidase (HRP) was used as the secondary antibody to incubate, and then immunoblotting chemiluminescence reagent ( ECL), autoradiography film exposure and film processing.
[0049] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com