Exendin-4 fusion protein, preparation method and uses thereof
A fusion protein and sequence technology, applied in the fields of peptide/protein components, chemical instruments and methods, hybrid peptides, etc., can solve the problems of insufficient safety and insufficient stability of fusion proteins, achieve high clinical application value, improve blood sugar Level control, the effect of prolonging half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Embodiment 1 Design of E4F4 fusion protein and construction of recombinant expression vector
[0067] Fusion protein sequence design, the mutant human IgG4Fc fragment ((aa.99-327) was fused to the C-terminal of two Exendin-4 fragments. At the same time, in order to realize the secretory expression of the fusion protein, a mouse signal was added to the N-terminal Peptide sequence, the final fusion protein obtained is: ssmIg-Ex-4-(G4S) 3 -Ex-4-(G4S) 3 -human mutant IgG4Fc (aa.99-327).
[0068] The fusion protein sequence containing signal peptide is as follows (SEQ ID No.6):
[0069] MGWSCIILFLVATATGVHSHGEGTFTSDLSKQMEEEAVRLFIEWLKNGGPSSGAPPPSGGGGSGGGGSGGGGSHGEGTFTSDLSKQMEEEAVRLFIEWLKNGGPSSGAPPPSGGGGSGGGGSGGGGSESKYGPPCPPCPAPEAAGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSQEDPEVQFNWYVDGVEVHNAKTKPREEQFNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKGLPSSIEKTISKAKGQPREPQVYTLPPSQEEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSRLTVDKSRWQEGNVFSCSVMHEALHNHYTQKSLSLSLG。
[0070] The corresponding coding n...
Embodiment 2
[0073] Example 2 Screening and identification of CHO stable expression strain (CHO-E4F4 cell)
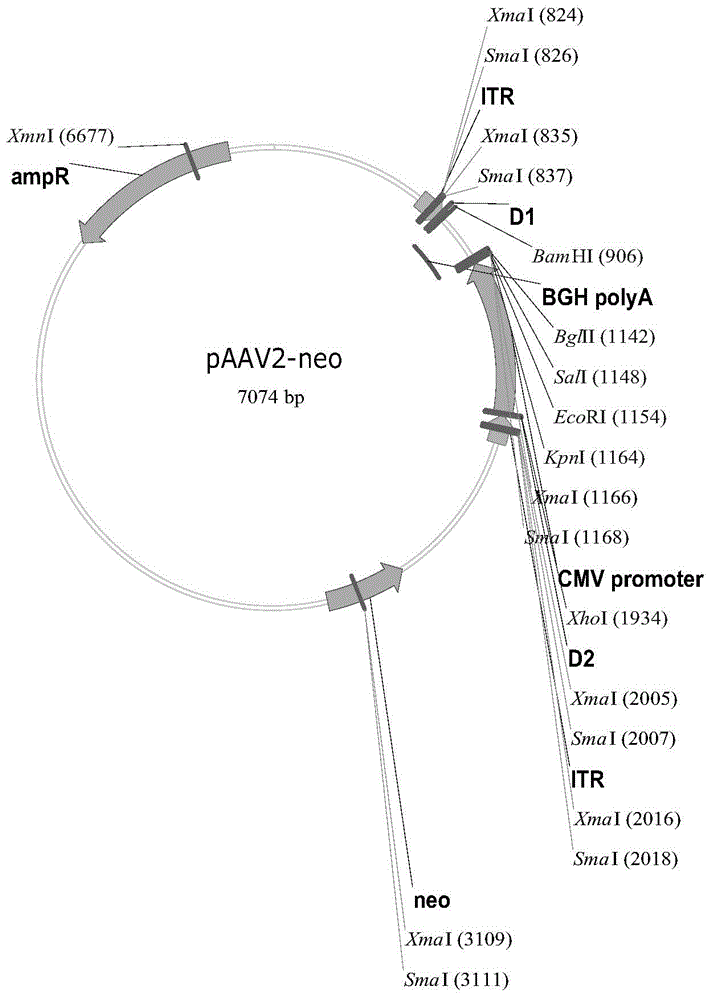
[0074] Take 10 μg of the pAAV2-E4F4 plasmid prepared in large quantities in Example 1, and use liposome Lipofectamine 2000 (purchased from Invitrogen, USA) to transfect CHO cells (purchased from ATCC, USA). The expression of the fusion protein in the culture supernatant was detected by ELISA. After four rounds of screening, high-expression E4F4 cells were selected, and the cells with stable expression were named CHO-E4F4 cells.
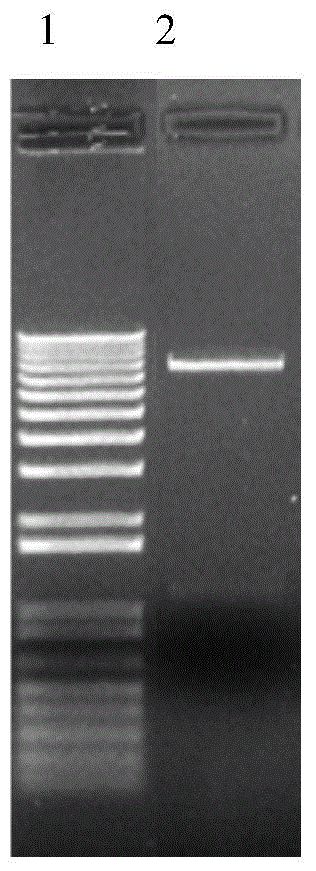
[0075] The RNA of CHO-E4F4 cells was extracted, and the transcription of the inserted fragment was identified by RT-PCR. The result is as image 3 As shown, CHO-E4F4 is a stable cell line that correctly expresses E4F4 recombinantly.
Embodiment 3
[0076] Example 3 Preparation of rhE4F4 fusion protein
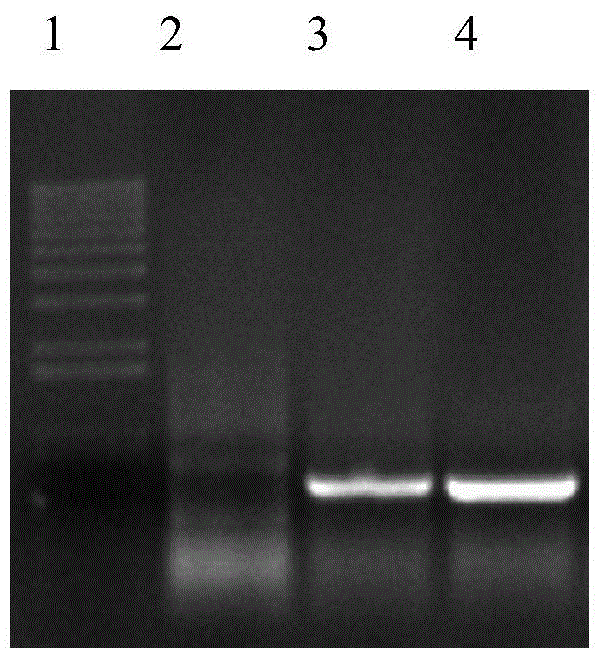
[0077] CHO-E4F4 cells were cultivated on a large scale, and the supernatant was purified by Protein A Sepharose F.F. (purchased from GE, USA) affinity chromatography column. The electrophoretic pattern of the purified protein is shown in Figure 4 , the purity can reach more than 90%.
[0078] Specific steps are as follows:
[0079] (1) Pretreatment of the expression supernatant: Slowly adjust the pH of the supernatant to 7.2 with 1M NaOH, centrifuge at 4000 rpm for 10 min at 4°C, and collect the supernatant.
[0080] (2) The centrifuged supernatant was passed through a Protein A Sepharose F.F. affinity chromatography column, and the fusion protein was washed with 0.1M citrate buffer (pH3.0), and the washed fusion protein was adjusted to pH 7.2 with 1M NaOH.
[0081] (3) The obtained rhE4F4 fusion protein was dialyzed with 10 mM phosphate buffer and stored at -20°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com