Vanadium-dependent haloperoxidase gene and its encoded protein in Laminaria
A technology of peroxidase and vanadium ions, which is applied in the fields of oxidoreductase, plant genetic improvement, genetic engineering, etc. It can solve the problems of lack of vanadium-dependent haloperoxidase clones and unconfirmed functions, and achieve high iodine The effect of substituting peroxidase activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1: Preparation of protein encoded by SjvIPO1 sequence
[0016] The full-length sequence of SEQ ID NO:1 was synthesized by Shanghai Xuguan Biotechnology Development Co., Ltd., and the recovered target fragment was connected to the cloning vector pMD19-T in a metal bath at 16°C overnight, and transformed into E. coli competent cells E.coli Top10 , spread on LB solid medium containing 100mg / mL Amp, culture overnight at 37°C, and select 4-10 positive clones for sequencing after IPTG / X-gal blue-white screening. Sequencing results were compared to isolate the HPO gene of Laminaria, named SjvIPO1. The full length of the SjvIPO1 CDS sequence is 1905bp, and its nucleotide sequence is shown in SEQ ID NO: 1, encoding 634 amino acids, with ATG as the start codon and TAA as the stop codon.
[0017] After the kelp SjvIPO1 PCR product was detected by 1% agarose gel electrophoresis, the target band was excised under ultraviolet light, recovered from the agarose gel, and the re...
Embodiment 2
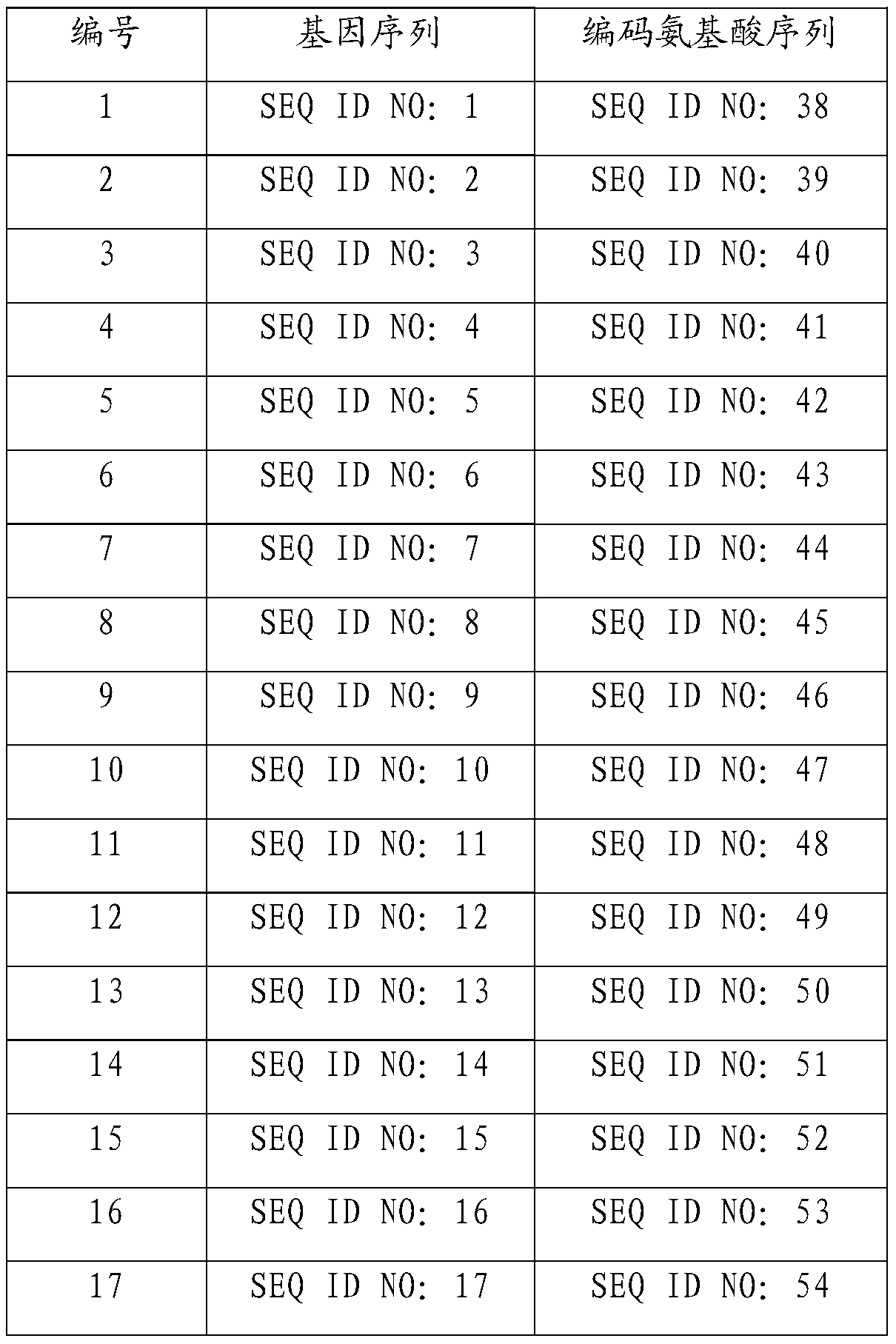
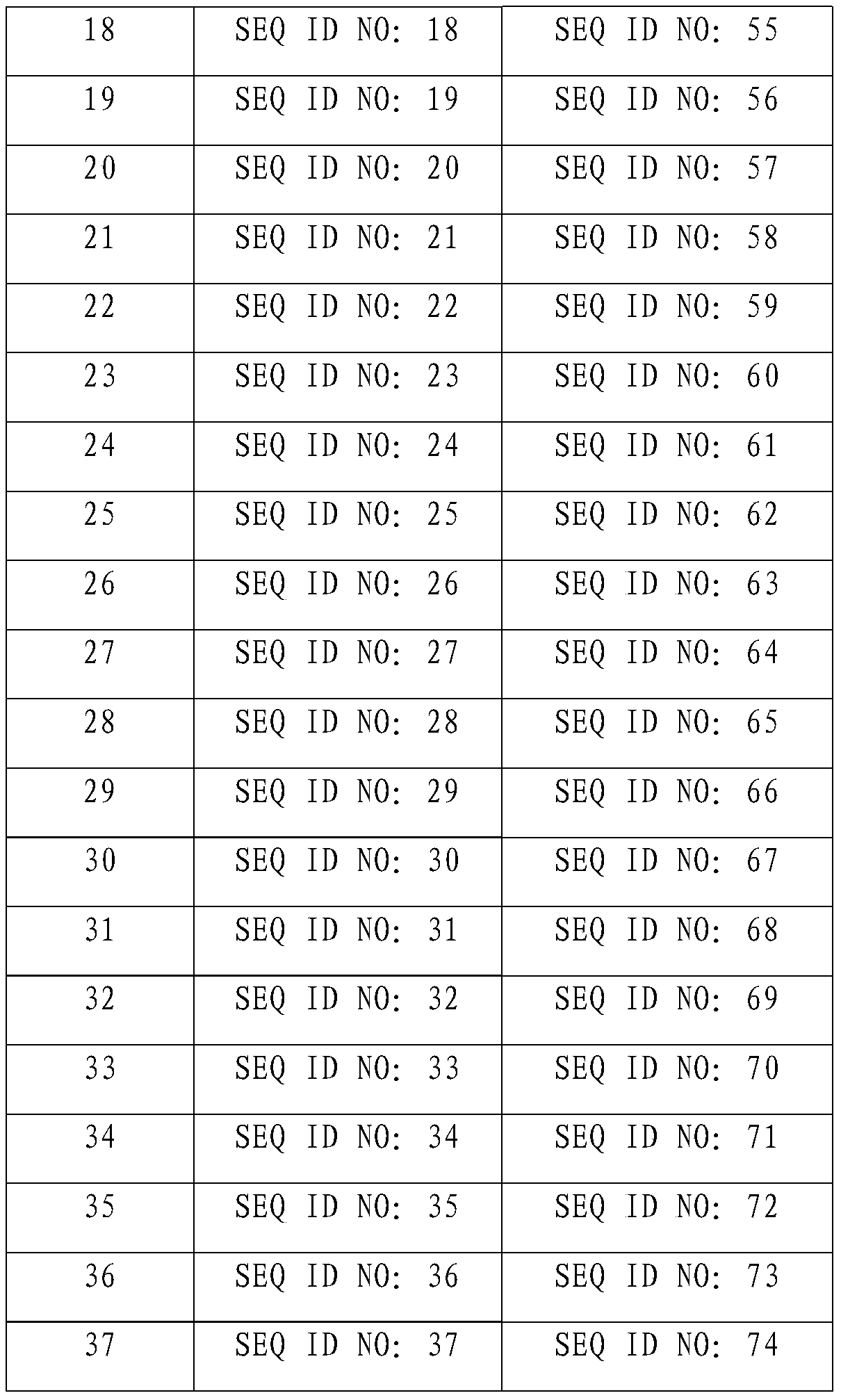
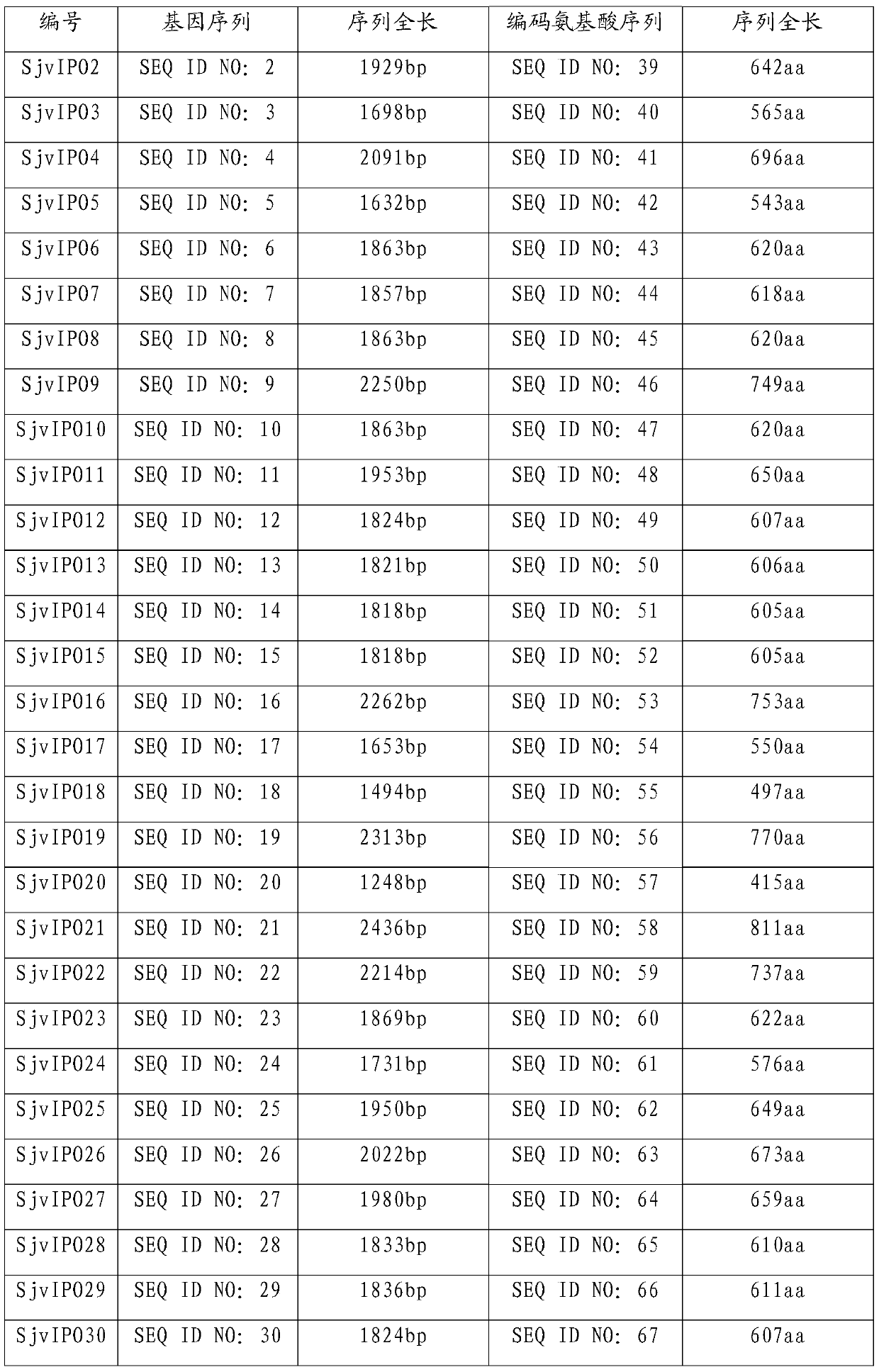
[0021] According to the method of Example 1, the protein encoded by SjvIPO2-SjvIPO37 (nucleotide sequence shown in SEQ ID NO: 2-37) was prepared, and the sequence of the prepared protein was shown in SEQ ID NO: 39-74 after sequencing, details as follows:
[0022]
[0023]
Embodiment 3
[0024] Example 3: Functional verification of SjvIPO1-37 encoded protein
[0025] The detection of SjvIPO1 protein activity adopts the standard method of monochlorodimedone (MCD). The enzyme activity detection reaction is as follows: Dilute the SjvIPO1 protein to 0.1 mM with PBS (dilute 100 microliters to 500 microliters), add 56 microliters of 10 mM Na 3 VO 4 , mixed at low speed overnight. 50mM MES (pH 6.50), 200mM KBr (or KCl, KI), 0.1mM MCD (monochlorodimedone) and appropriate amount of Na 3 VO 4 Treated recombinant SjvIPO1 protein, add 1mM H 2 o 2 initial reaction. Remove the above H 2 o 2 The system was mixed and incubated at the corresponding temperature for 2 minutes before starting the reaction. Using the corresponding buffer as a blank control, the content of MCD in the liquid phase was measured at 278 nm by high performance liquid chromatography, and 4 parallel samples were set for each reaction. . After testing, using I - The enzyme activity is 5895U / mg, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com