Inositol preparing method
A technology of myo-inositol and myo-inositol monophosphatase, applied in biochemical equipment and methods, lyases, transferases, etc., can solve problems such as high cost and low yield, and achieve low production cost, high yield and pollution. low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0058] Experimental example 1 in vitro multi-enzyme catalyzed conversion of starch into inositol
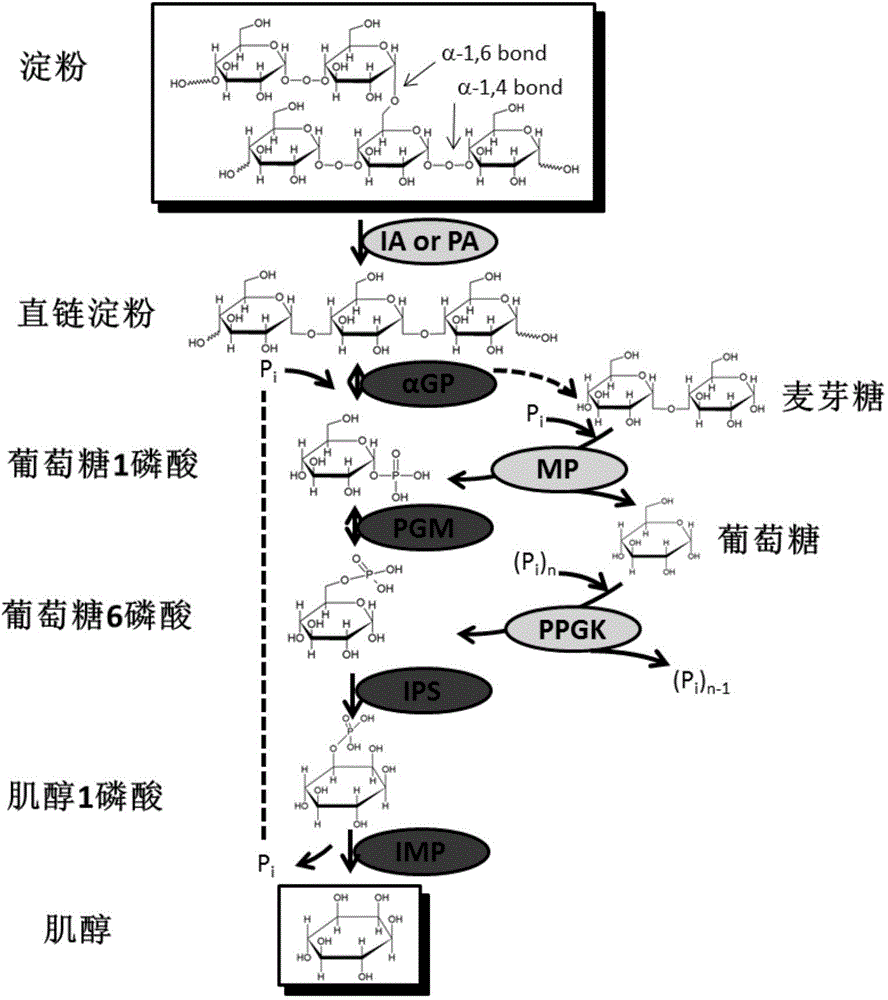
[0059] Starch was converted to inositol by an in vitro multi-enzyme catalytic system ( figure 1 ). These key enzymes include: (1) Glucan phosphorylase (αGP, EC 2.4.1.1), which releases glucose-1-phosphate from starch; (2) Glucose phosphomutase (PGM, EC 5.4.2.2) , catalyzing glucose-1-phosphate to glucose-6-phosphate; (3) inositol-3-phosphate synthase (IPS, EC 5.5.1.4), which catalyzes glucose-6-phosphate to inositol-3-phosphate; ( 4) Inositol monophosphatase (IMP, EC 3.1.3.25), which dephosphorylates inositol-3-phosphate to inositol. Since the last two enzyme reactions are irreversible reactions, the enzyme-catalyzed system can obtain a high conversion rate.
[0060] In the present invention, the glucan phosphorylase is derived from Thermotoga maritima, and the number of the gene on KEGG is TM1168, and the phosphomutase of glucose is also derived from Thermotogamaritima, and ...
experiment example 2
[0064] Experimental example 2 in vitro multi-enzyme catalyzed conversion of starch into inositol
[0065] The preparation of glucan phosphorylase, glucose phosphomutase, inositol-3-phosphate synthase and inositol monophosphatase was the same as that in Experimental Example 1.
[0066] In a 0.75 ml reaction system containing 100 mM HEPES buffer (pH7.2), 10 mM inorganic phosphate, 5 mM divalent magnesium ion, 0.5 mM zinc ion, 0.05 U / mL dextran phosphorylase, 1U / mL glucose phosphomutase, 0.05U / mL inositol-3-phosphate synthase and 2U / mL inositol monophosphatase, 10g / L soluble starch, catalyzed reaction at 40℃, 40 reactions Hour.
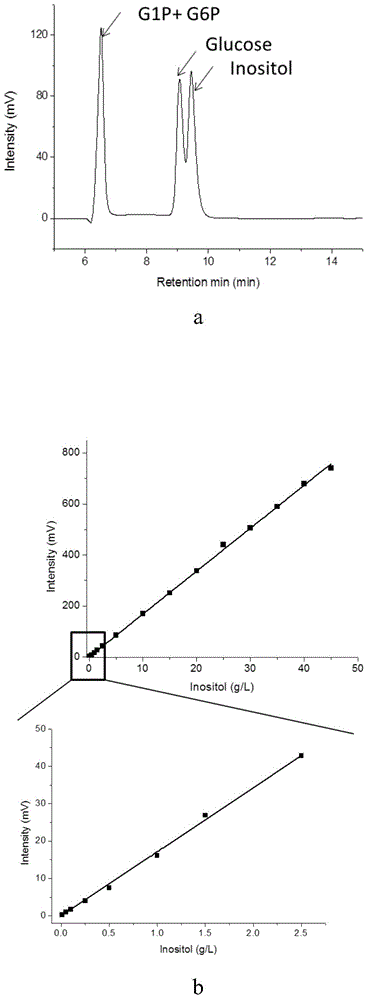
[0067] After the reaction, the final concentration of inositol was 0.9g / L, and the conversion rate was 9%.
experiment example 3
[0068] Experimental example 3 in vitro multi-enzyme catalyzed conversion of starch into inositol
[0069] The preparation of glucan phosphorylase, glucose phosphomutase, inositol-3-phosphate synthase and inositol monophosphatase was the same as that in Experimental Example 1.
[0070] In a 0.75 ml reaction system containing 100 mM HEPES buffer (pH7.2), 10 mM inorganic phosphate, 5 mM divalent magnesium ion, 0.5 mM zinc ion, 0.05 U / mL dextran phosphorylase, 1U / mL glucose phosphomutase, 0.05U / mL inositol-3-phosphate synthase and 2U / mL inositol monophosphatase, 10g / L soluble starch, catalyzed reaction at 80℃, 40 reactions Hour.
[0071] After the reaction, the final concentration of inositol was 3.6g / L, and the conversion rate was 36%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com