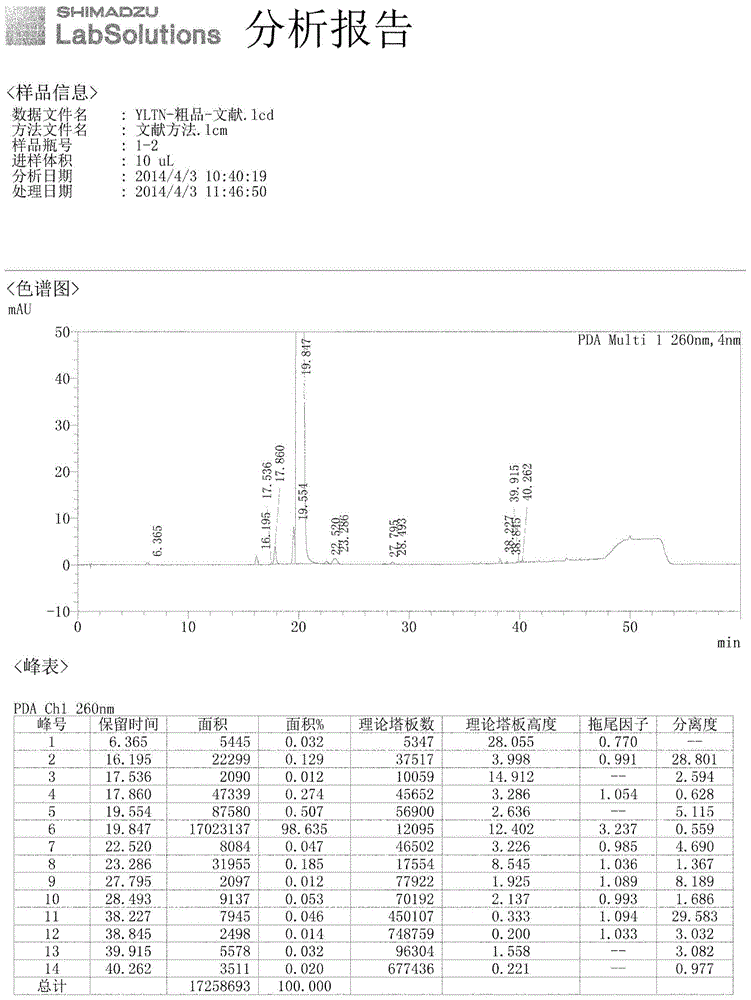
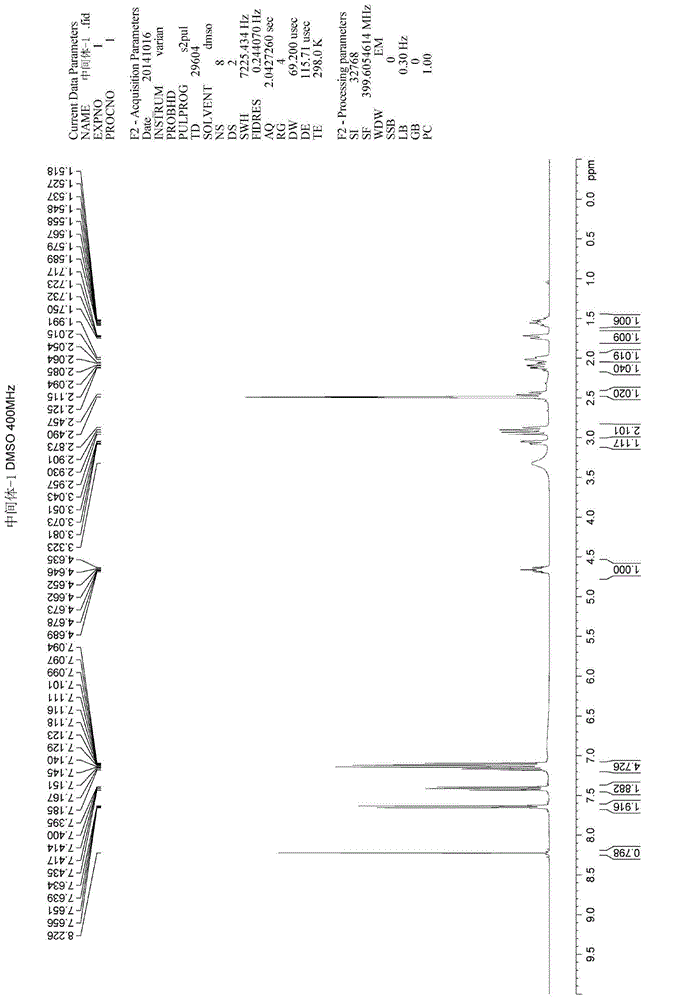
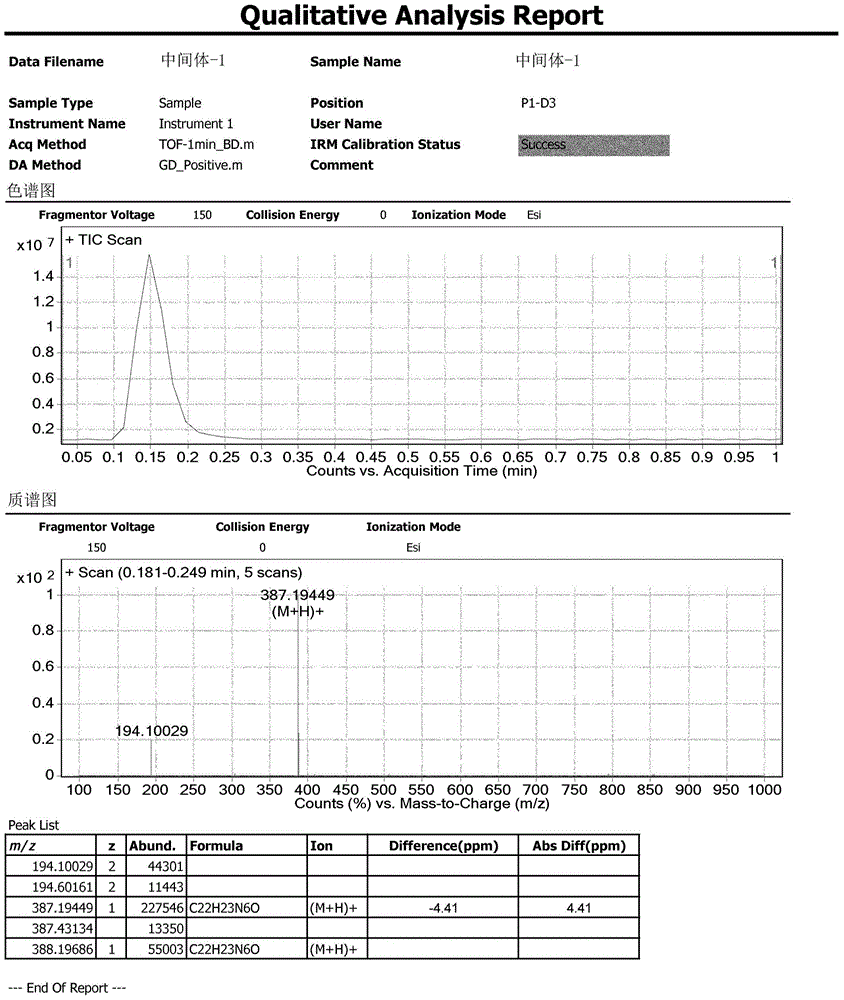
HPLC method for analyzing ibrutinib and ibrutinib-preparation-related substances and usage of impurities as reference standard
A technology of ibrutinib and impurities, applied in the field of drug synthesis, can solve the problems of not being able to truly reflect the quality of the drug, not being able to accurately measure the real content of impurities, and not having qualitative analysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] Intermediate-1: (R)-3-(4-phenoxyphenyl)-1-(piperidin-3-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine synthesis
[0118] Add 155mL tetrahydrofuran to a 500mL reaction flask, and add 3-(4-phenoxyphenyl)-1H-pyrazol[3,4-D]pyrimidin-4-amine (SM1) (5g, 1eq) sequentially under stirring , (S)-1-tert-butoxycarbonyl-3-hydroxypiperidine (SM2) (4.97g, 1.5eq), triphenylphosphine (13g, 3.0eq). Under temperature control at 25° C., a solution of diisopropyl azodicarboxylate in tetrahydrofuran (dissolve 10 g, 3.0 eq of diisopropyl azodicarboxylate in 10 mL of tetrahydrofuran) was added dropwise within 30 minutes. After the dropwise addition was completed, the temperature was controlled at 25° C., and the reaction was continued for 5 hours (TLC monitoring: ethyl acetate:methanol=10:1). Stirring and distillation under reduced pressure. The temperature was controlled at 15°C, and 30 mL of concentrated hydrochloric acid was added dropwise to the residue for 30 minutes. After the addition was c...
Embodiment 2
[0119] Embodiment 2: Preparation of Ibrutinib
[0120] Under nitrogen protection, 50 mL of dichloromethane, Intermediate-1 (5 g, 1 eq), and N, N-diisopropylethylamine (2 g, 1.2 eq) were sequentially added into a 100 mL three-necked flask. Under the condition of temperature control at -10°C, start to add the dichloromethane solution of acrylic anhydride (1.96g, 1.2eq) dropwise for 30 minutes. Until the reaction of the raw materials was complete (TLC detection, methanol: ethyl acetate: triethylamine = 1:5:0.05). The reaction solution was washed with 200 mL of 5% citric acid aqueous solution, the water phase was removed, concentrated and evaporated to remove dichloromethane. The residue was recrystallized three times with isopropanol to obtain the finished product of ibrutinib: 3.63 g. 1 H-NMR (400Mz, DMSO-d 6 ( m, 1H), 5.570~5.713(m, 1H), 4.690~4.716(m, 1H), 4.554~4.583(m, 0.5H), 4.208(m, 1H), 4.052~4.085(m, 0.5H), 3.674~3.731(m, 0.5H), 3.184~3.214(m, 1H), 2.972~3.027(m, 0....
Embodiment 3
[0121] Example 3: Impurity A: (R)-1-(3-(4-amino-3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl Synthesis of ]piperidin-1-yl)-3-chloropropyl-1-one.
[0122] Under nitrogen protection, 50ml of dichloromethane was added to a 100mL three-necked flask, and Intermediate-1 was added sequentially under stirring: (R)-3-(4-phenoxyphenyl)-1-(piperidin-3-yl )-1H-pyrazol[3,4-d]pyrimidin-4-amine (YLTN-1) (1.00g, 1eq), N, N-diisopropylethylamine (0.40g, 1.2eq), cooled to -20~-10°C, start to add 3-chloropropionyl chloride (1.46g, 1eq) dropwise. After the dropwise addition, the solution turns from turbid to clear. Continue to stir for 20-30 minutes. LC-MS detects that the raw materials disappear, and distills under reduced pressure. , dichloromethane was distilled until no fraction was distilled off, and the crude product was purified by column chromatography, the elution ratio was: methanol: ethyl acetate=1:10, a total of 400 mL of the eluent was collected, distilled under reduced pres...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com