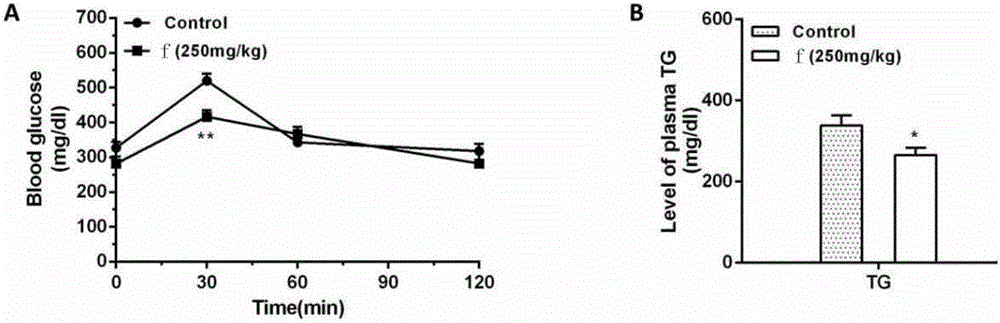
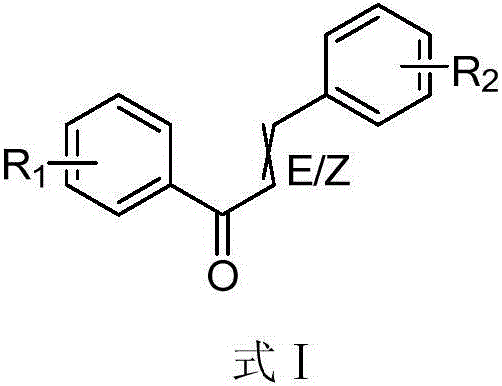
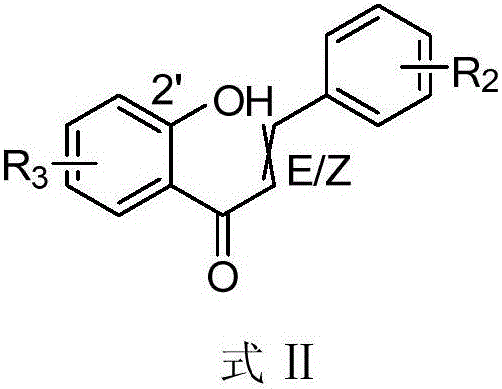
Novel applications of chalcone compound
A technology of chalcones and compounds, applied in the field of medicine, can solve problems such as lack, lack of triglyceride regulation, hypoglycemia, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Preparation of 2-methoxyl-3'-hydroxyl-chalcone a
[0032]
[0033]Dissolve 3'-hydroxyacetophenone in absolute ethanol (10mg / mL), add 2-methoxybenzaldehyde (1eq.), and slowly add 40% KOH aqueous solution dropwise under stirring at 0°C, and stir overnight at room temperature. The remaining raw materials were extracted with ether, the aqueous layer was acidified with 2M hydrochloric acid, the solid precipitated out, extracted with ethyl acetate, dried and concentrated over anhydrous sodium sulfate, and recrystallized from ethanol to obtain 2-methoxy-3′-hydroxy-chalcone a (yellow solid, 84%). And NMR and mass spectrometry were performed on a, the results are as follows: 1 HNMR (300MHz, CDCl 3 )δ: 8.12(d, J=15.9Hz, 1H), 7.62(t, J=6.3Hz, 1H), 7.59(d, J=15.9Hz, 1H), 7.55(m, 2H), 7.35-7.41( m,2H),7.07(dd,J 1 =7.8Hz,J 2 =2.1Hz,1H),6.91-7.02(m,2H),3.92(s,3H).ESI-MS:[M+H]+:255.1.
Embodiment 2
[0034] Example 2 Preparation of 4-methoxy-2′,6′-dihydroxy-chalcone b
[0035]
[0036] (1). Dissolve 2′,6′-dihydroxyacetophenone (350 mg, 2.30 mmol) in 20 mL of anhydrous dichloromethane, and add N,N-diisopropylethylamine ( 0.76 mL, 4.60 mmol) and chloromethyl methyl ether (0.26 mL, 3.45 mmol), stirred for 4.5h. Add 20 mL of ethyl acetate, and wash with saturated sodium bicarbonate solution, 30 mL of water and 30 mL of saturated brine successively. The organic layer was dried over anhydrous sodium sulfate, concentrated, and column chromatography (petroleum ether / ethyl acetate=20:1~15:1) gave 2′-hydroxyl-6′-methoxymethyleneoxyacetophenone ( Colorless oily liquid, 440 mg, yield 97.8%).
[0037] (2). 2'-hydroxy-6'-methoxymethyleneoxyacetophenone (200 mg, 1.02mmol) and 4-methoxybenzaldehyde (153mg, 1.12mmol) were dissolved in 10mL of ethanol, After cooling to 0°C, 10 mL of 40% potassium hydroxide aqueous solution was added dropwise. After stirring at room temperature for 18...
Embodiment 3
[0039] Example 3 The preparation of 2-methoxy-2', 3'-dihydroxy-chalcone c:
[0040]
[0041] In Example 2 (1), 2', 6'-dihydroxyacetophenone is replaced with 2', 3'-dihydroxyacetophenone, and 2'-hydroxyl-3'-methoxyl group is obtained in (1) Methyleneoxyacetophenone (colorless oily liquid, 380mg, yield 84.5%); 2'-hydroxyl-3'-methoxymethyleneoxyacetophenone and 2-methoxybenzaldehyde were used for (2) react to get 2-methoxy-2'-hydroxyl-3'-methoxymethyleneoxy-chalcone (yellow solid, 250 mg, 78.0%); Methyl protection gave the product 2-methoxy-2',3'-dihydroxy-chalcone c (yellow solid, 110 mg, 91.2%). And c was characterized by NMR and mass spectrometry, the results are as follows: 1 HNMR (Acetone-d6) δ: 13.20(s, 1H), 8.33(d, J=15.6Hz, 1H), 8.03(d, J=15.6Hz, 1H), 7.94-7.92(m, 2H), 7.73( d, J=8.4Hz, 1H), 7.48(t, J=7.8Hz, 1H), 7.15(d, J=8.0Hz, 2H), 7.04(t, J=7.4Hz, 1H), 6.87(t, J=7.8Hz,1H),3.99(s,3H). 13 CNMR(Acetone-d6)δ:195.5,160.0,152.7,147.3,141.3,133.5,130.0,124.1,121.8,12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com