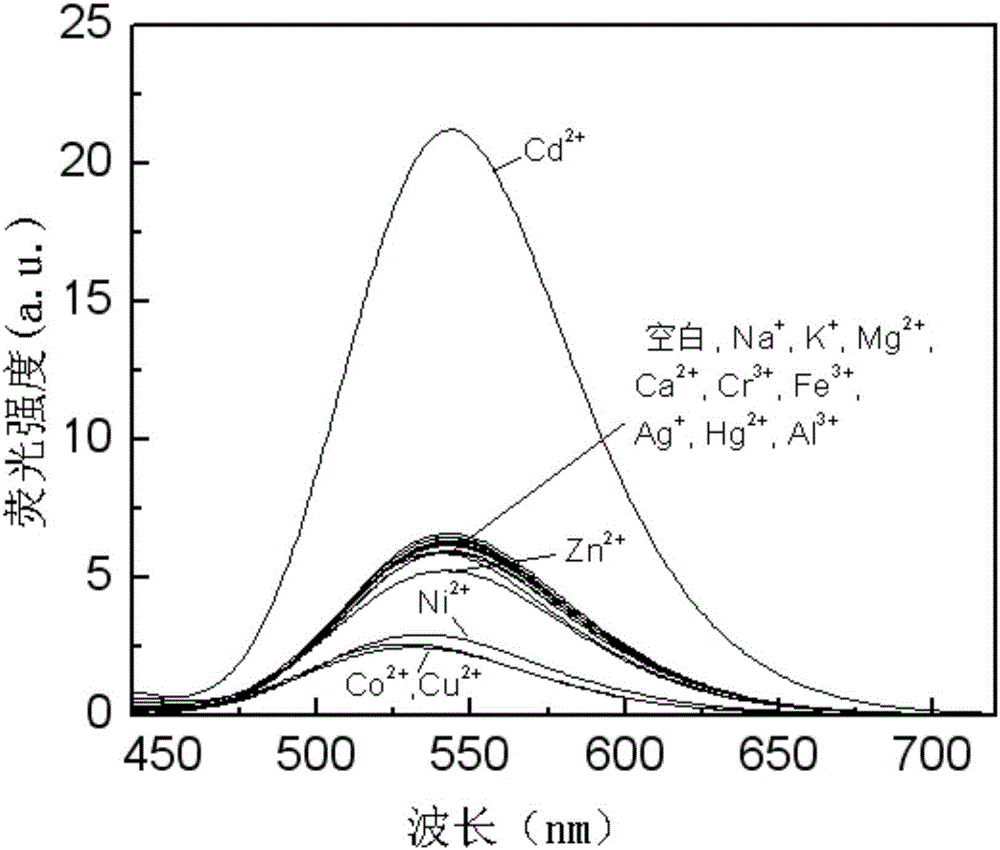
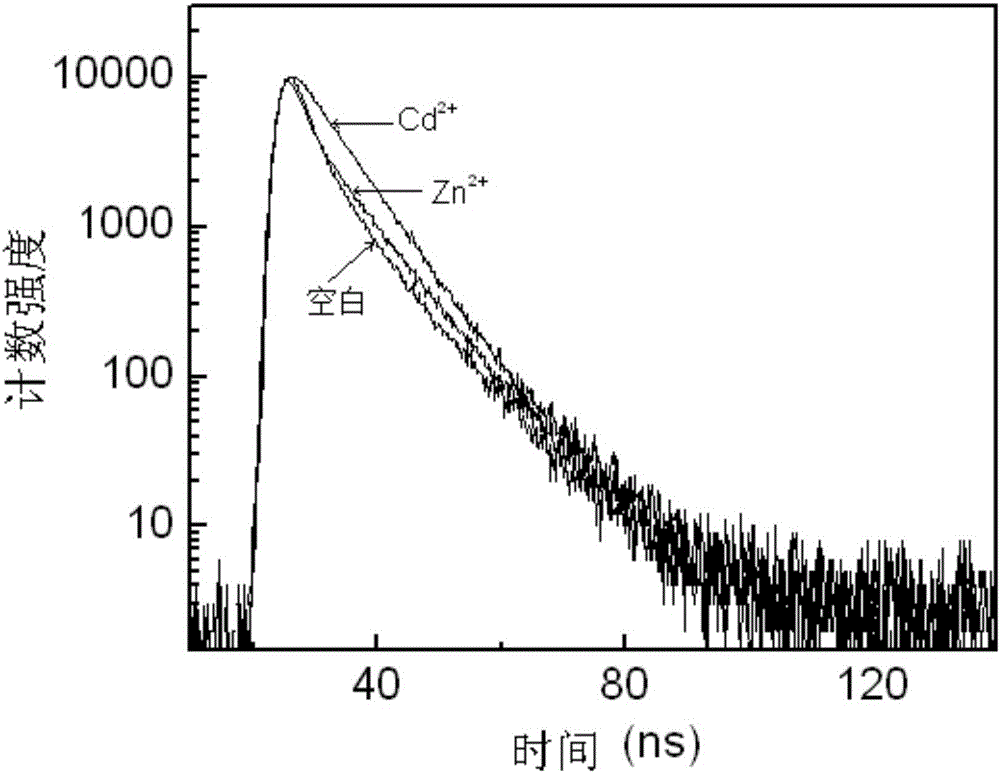
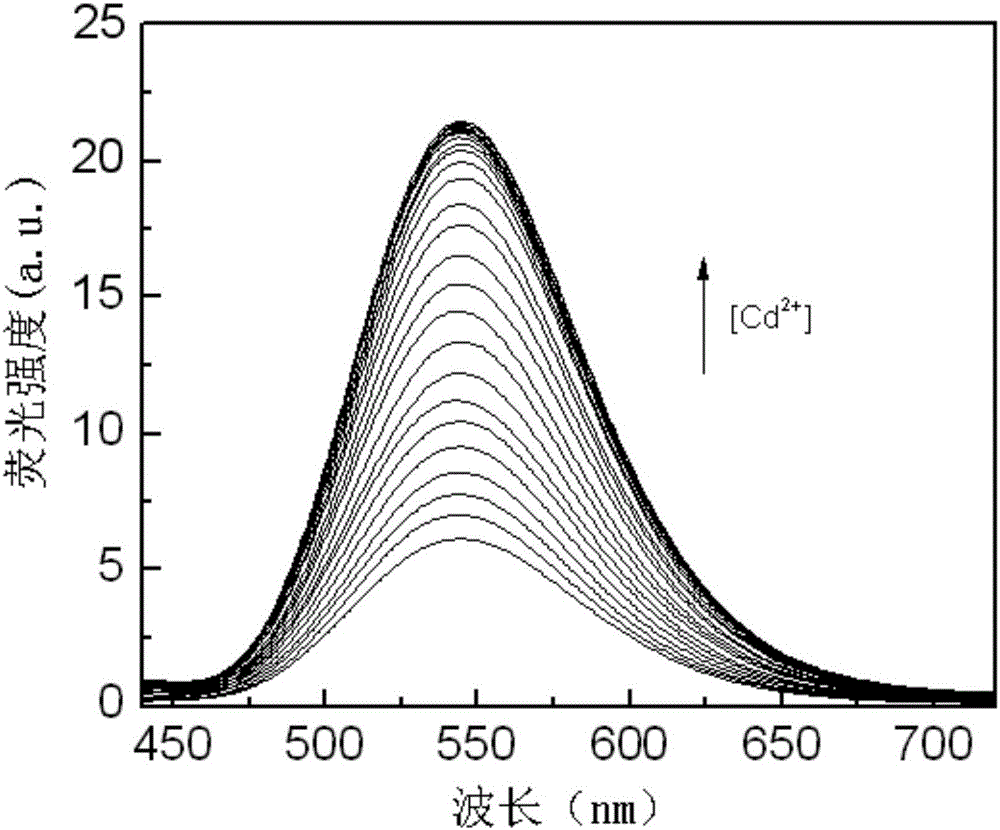
Aza-naphthyl imide Cd<2+> probe molecule, synthesis method therefor and application of aza-naphthyl imide Cd<2+> probe molecule
A technology of xinaphthimide and probe molecules, applied in chemical instruments and methods, analytical materials, fluorescence/phosphorescence, etc., can solve problems such as interference and poor interference, achieve high selectivity and sensitivity, and have a wide range of potential application values Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0024] Specific embodiment one: the azanaphthimide Cd of the present embodiment 2+ Probe molecule, its structural formula is:
[0025]
[0026] Wherein, said R is a C1-C4 linear alkyl group.
specific Embodiment approach 2
[0027] Specific embodiment two: the azanaphthimide class Cd described in specific embodiment one 2+ The synthetic method of probe molecule is carried out according to the following steps:
[0028] 1. Add pyrano[3,4,5-de]quinoline-2,4,6(1H)-trione and alkylamine to ethanol at a molar ratio of 1:(4.8~5.2) , heated to boiling and refluxed for 4-6 hours under the protection of nitrogen; the reaction solution was spin-dried, and then separated by silica gel column chromatography to obtain intermediate Ⅰ;
[0029] 2. Add intermediate I and phosphorus oxychloride obtained in step 1 to dioxane at a molar ratio of 1:(9.5-10.5), and react for 2-3 hours at 75-85°C; Ammonia water adjusts the pH value of the reaction solution to alkaline; the reaction solution is spin-dried, and then separated by silica gel column chromatography to obtain intermediate II;
[0030] 3. Add intermediate II and 2-aminobenzylamine obtained in step 2 to ethylene glycol methyl ether in a molar ratio of 1: (4.8-...
specific Embodiment approach 3
[0033] Specific embodiment three: the difference between this embodiment and specific embodiment two is: in step one, pyrano[3,4,5-de]quinoline-2,4,6(1H)-trione, alkylamine The molar ratio is 1:5. Others are the same as in the second embodiment.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com