Reactive near-infrared photoactive receptor and synthesis method and application thereof
A near-infrared light and synthesis method technology, applied in chemical instruments and methods, luminescent materials, fluorescence/phosphorescence, etc., can solve problems such as limiting effects, achieve low autofluorescence interference, strong tolerance, and improve selectivity and sensitivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] In a preferred embodiment of the present invention, there is also provided a method for preparing the above-mentioned reactive near-infrared photoelectrically active acceptor, the method comprising the addition reaction of silarhodamine spironolactone and ferroceneformyl isothiocyanate to generate The acceptor step.
[0039] The structural formula of silarhodamine spironolide hydrazide is:
[0040]
[0041] The structural formula of ferroceneformyl isothiocyanate is:
[0042]
[0043] Among them, silarhodamine spironolide hydrazide and ferroceneformyl isothiocyanate are existing substances that can be prepared by those skilled in the art through conventional methods, and there is no special limitation here. For example, silarhodamine spironolide hydrazide can be found in the literature (Mao, G.-J.; Liang, Z.-Z.; Bi, J.; Zhang, H.; Meng, H.-M.; Su, L.; Gong, Y.-J.; Feng, S.; Zhang, G. Anal. Chim. Acta 2019, 1048, 143-153.) method prepared; ferrocene formyl isothi...
Embodiment 1
[0066] synthesis of receptors
[0067] Equipped with stirring magnet, constant pressure dropping funnel and N 2 Add silarhodamine spironolide hydrazide (0.443g, 1.0mmol) and DMF (5mL) into the three-necked flask of the catheter, and then add ferroceneformyl isothiocyanate (1.356g, 5.0mmol) in DMF (3mL) dropwise. ) solution. Thin-layer chromatography tracking, after the reaction, the reaction solution was poured into water, extracted with dichloromethane, the extract was washed with saturated brine, anhydrous MgSO 4 Dry, filter off the desiccant, evaporate the filtrate to remove the solvent under reduced pressure, and the residue is separated by column chromatography (ethyl acetate / petroleum ether=1:3, R f =0.3), obtain orange solid powder (acceptor of the present invention) 0.594g, productive rate is 83.3%, m.p.182-183 ℃.IR (cm –1 ): v max 3333(N-H), 1711, 1655(C=O), 1349(C=S). 1 HNMR (300MHz, CDCl 3 ,TMS):δ11.42(s,1H),8.41(s,1H),8.02-8.00(m,1H),7.46-7.44(m,2H),6.98-6.8...
Embodiment 2
[0069] The recognition effect of receptors on various tested ions in the ultraviolet-visible spectrum detection embodiment 1
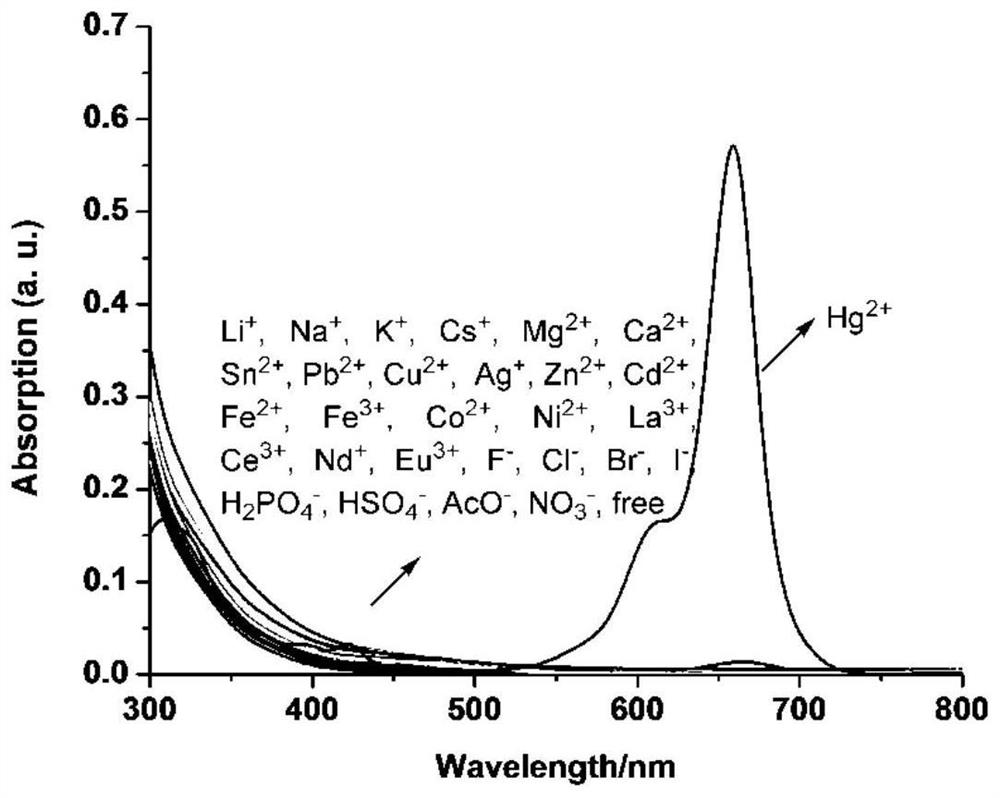
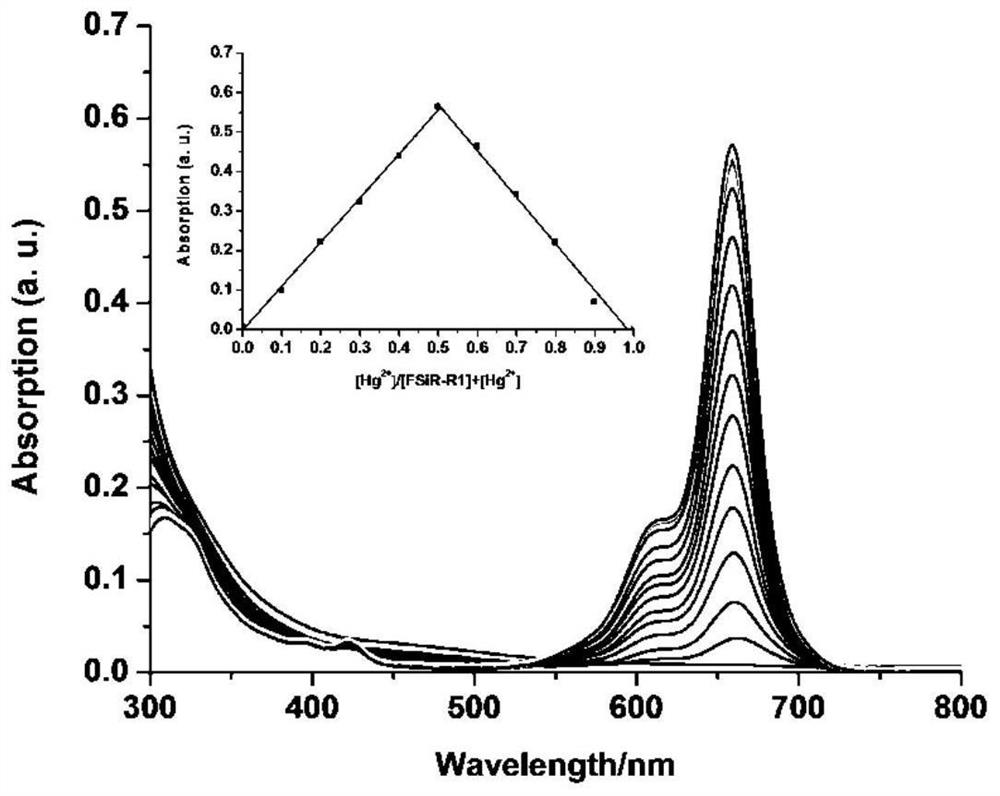
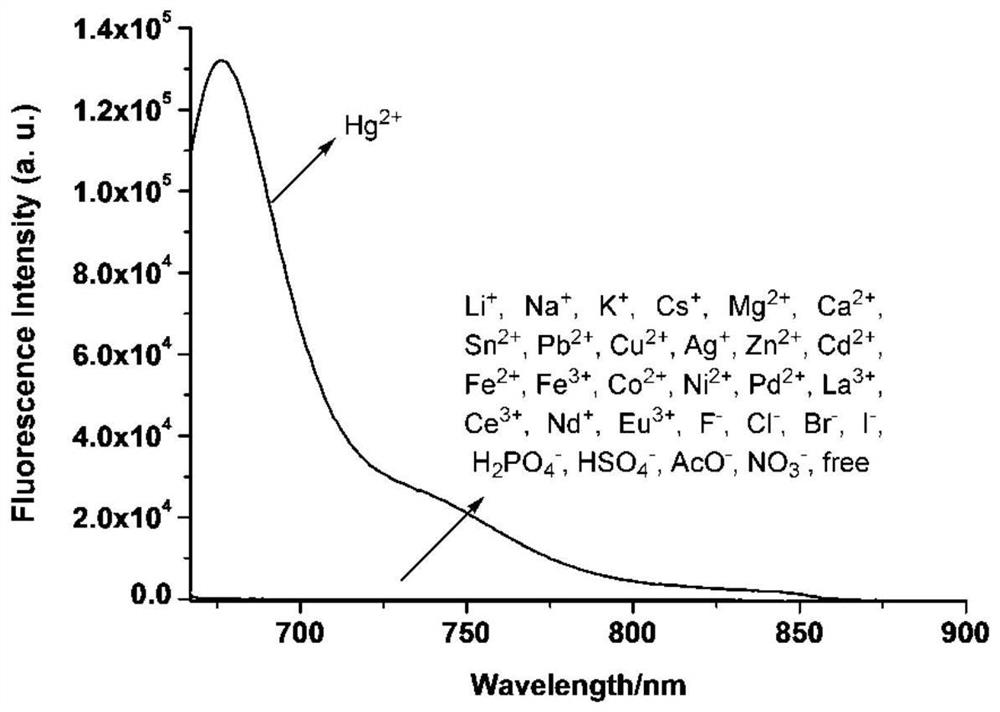
[0070] Prepare 5.0×10 in a 10mL volumetric flask -4 Receptor standard solution of M, respectively prepare 1.0×10 in 10mL volumetric flask -3 Different ion aqueous solutions of M to be tested; respectively pipette 100 μL of 5.0×10 -4 The acceptor standard solution of M and 50 μL of the above-mentioned ion aqueous solution to be tested were adjusted to 5 mL with 0.5% acetone-water solution, and the ultraviolet-visible absorption intensities were measured respectively.
[0071] The result is as figure 1 , indicating that only the addition of Hg 2+ Ions, the UV-visible absorption of the acceptor changes significantly, and a new strong UV-visible absorption peak appears at 659nm, which is caused by the opening of the spiro ring in the structure of sila rhodamine, while the addition of other ions is not obvious change, so the receptor can recognize Hg wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com