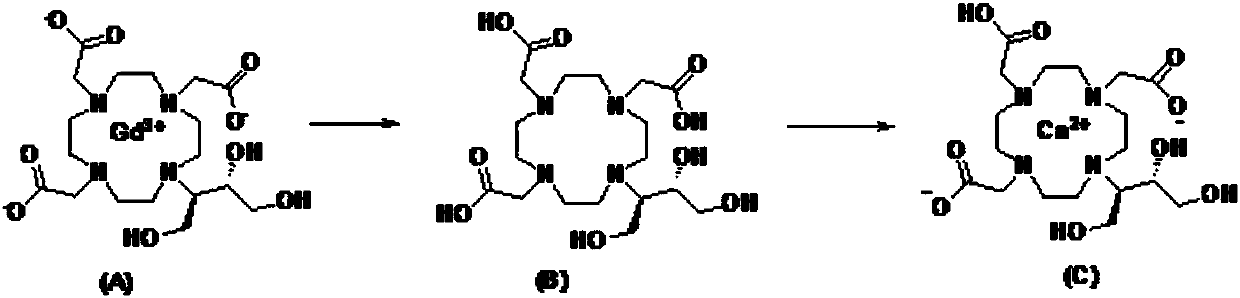
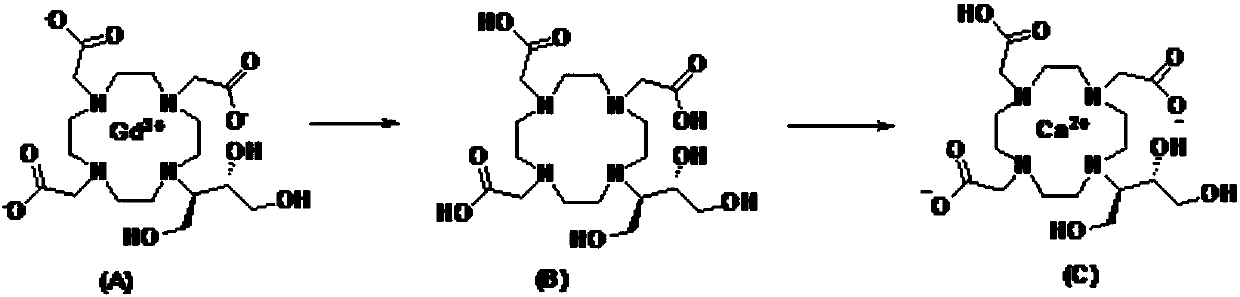
Preparation method of high-purity kobutrol calcium
A high-purity technology of calbutril calcium, applied in the field of medicine, can solve problems such as difficulty in obtaining high-purity ligands, failure to obtain ligand crystals, large industrial wastes, etc., to achieve easy industrial application, reduce the generation of wastes, and clear routes Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] At room temperature, take 10 g of gadobutrol (purity ≥ 99%) and add it to the reactor, add 100 ml of purified water and stir to dissolve, add 14.9 g of oxalic acid, heat up to 95 ° C and stir for 6 h to remove gadolinium, cool to room temperature, filter, add 16.5 g of the filtrate g calcium carbonate was refluxed for 5 hours, cooled to room temperature and filtered, the filtrate was added with 1500mL of ethanol to reflux and crystallized for 3 hours, cooled to room temperature, filtered out the crystals, rinsed with an appropriate amount of ethanol, and the filter cake was vacuum-dried at 50°C for 5 hours to obtain Cobutrol Calcium 6.2g, yield 78%, HPLC detection purity 99.8%.
Embodiment 2
[0041] At room temperature, add 50 g of gadobutrol (purity ≥ 99%) into the reactor, add 500 ml of purified water and stir to dissolve, add 74.5 g of oxalic acid, raise the temperature to 90° C. and stir to remove gadolinium for 6 hours. Cool to room temperature, filter, add 82.6g of calcium carbonate to the filtrate to reflux for 5 hours, cool to room temperature and filter, add 7500mL ethanol to the filtrate to reflux and crystallize for 3 hours, cool to room temperature, filter out the crystals, rinse with an appropriate amount of ethanol, and place the filter cake at 50°C After vacuum drying for 5 hours, 33.5 g of Calcium Cobutrol was obtained with a yield of 82.9% and a purity of 99.5% by HPLC.
Embodiment 3
[0043] At room temperature, take 50g of gadobutrol (purity ≥ 99%) into the reactor, add 500ml of purified water and stir to dissolve, add 150g of oxalic acid, heat up to 100°C and stir for 6 hours to remove gadolinium, cool to room temperature, filter, add 82.6g of filtrate Calcium carbonate was refluxed for 5 hours, cooled to room temperature and filtered, and 7500 mL of ethanol was added to the filtrate to reflux for crystallization for 3 hours, cooled to room temperature, the crystals were filtered out, rinsed with an appropriate amount of ethanol, and the filter cake was vacuum-dried at 50°C for 5 hours to obtain 32.1 g, yield 79.5%, the HPLC detection purity is 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com