Composition of multiple isoflavone derivatives, preparing method and medical application
A technology of derivatives and compositions, applied in the directions of drug combinations, compounds of Group 5/15 elements of the periodic table, chemical instruments and methods, etc., can solve the problems of unsatisfactory hypoglycemic effect, etc., and achieve good hypoglycemic effect. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
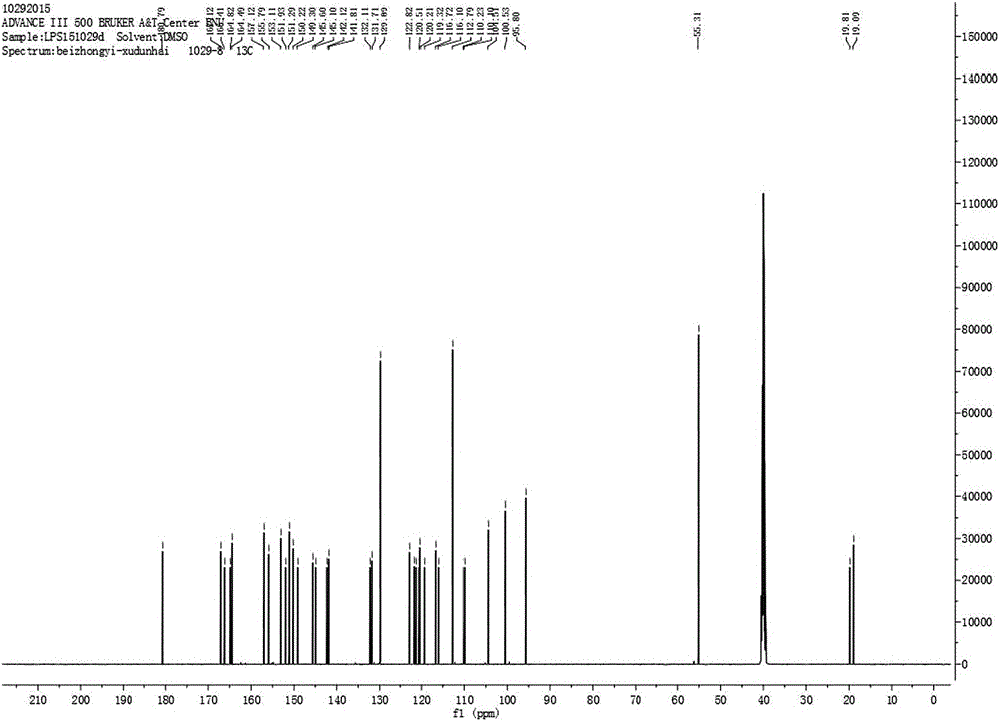
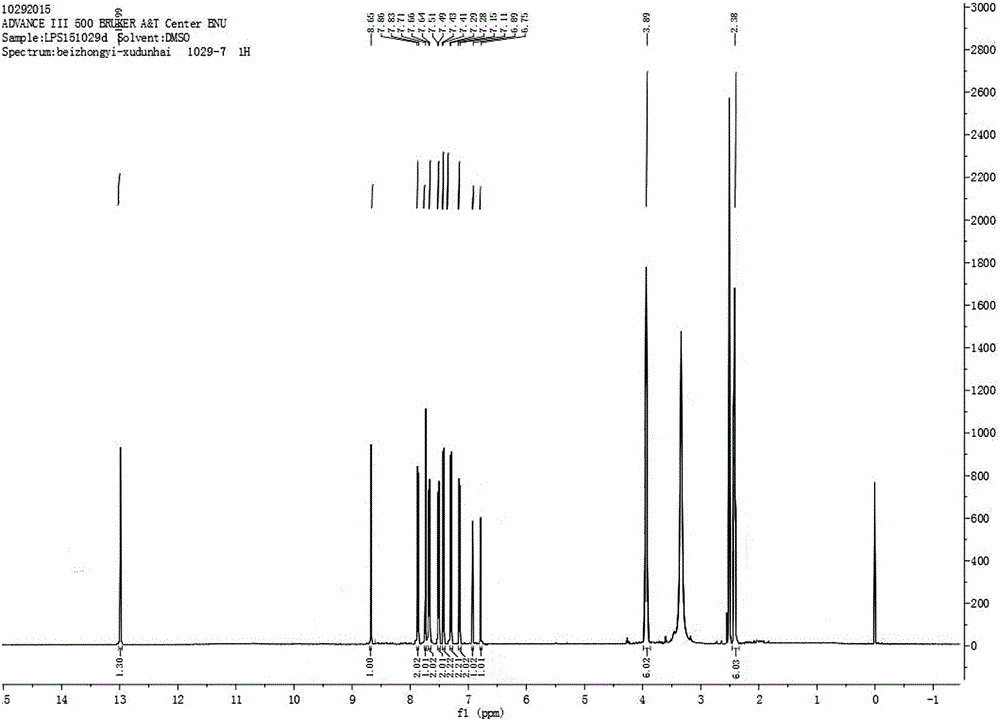
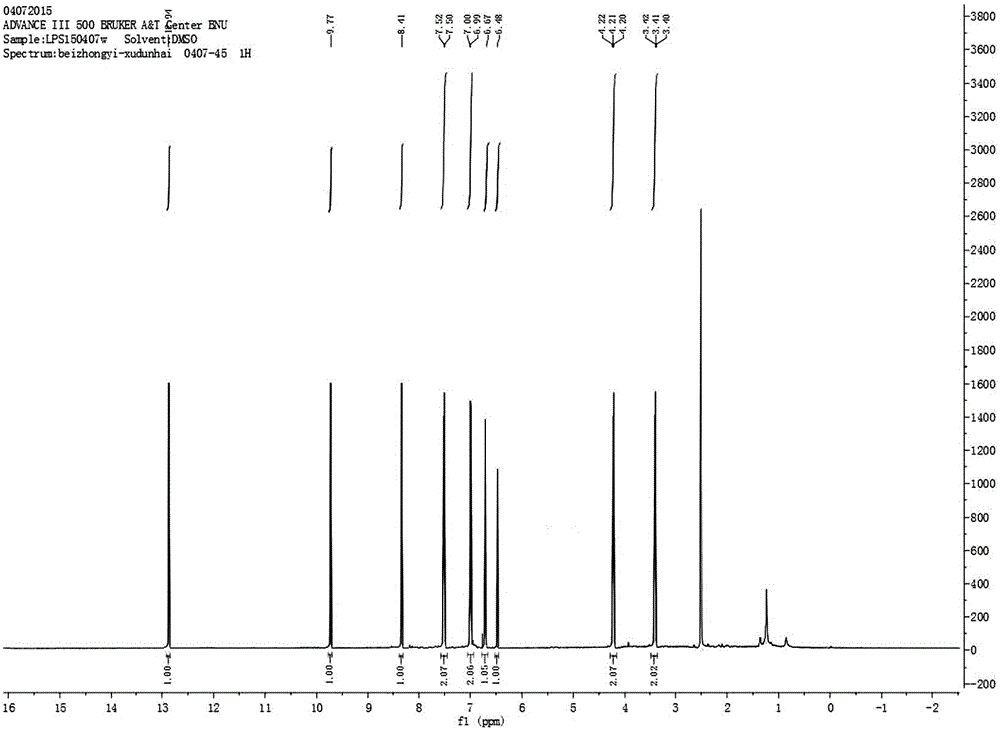
[0028] Preparation of 4′,7-diacetylisoferulic acid genistein:
[0029] a) Preparation of acetyl isoferulic acid:
[0030] 1. Add 1g isoferulic acid and 20mL acetyl chloride into a 100mL round bottom flask;
[0031] 2. Place in an ice bath to lower the temperature of the system to 0°C, then add 5 drops of anhydrous pyridine dropwise;
[0032] 3. Heat up to 110°C under stirring, and reflux for 3 hours;
[0033] 4. After cooling at room temperature, a large amount of solids appeared, suction filtered, and the filter residue was washed with water and absolute ethanol in sequence, and dried to obtain the crude product of acetylisoferulic acid;
[0034] 5. The crude product is eluted with 300-400 mesh silica gel, and the elution condition is petroleum ether: ethyl acetate: methanol: acetic acid = 300:100:50:10 to obtain acetyl isoferulic acid monomer.
[0035] b) Preparation of 4′,7-diacetylisoferulic acid genistein:
[0036] 1. Weigh 0.3g of genistein, 0.1g of acetylisoferulic ...
Embodiment 2
[0069] Preparation of 4′,7-diacetylisoferulic acid genistein:
[0070] a) Preparation of acetyl isoferulic acid:
[0071] 1. Add 1g isoferulic acid and 20mL acetyl chloride into a 100mL round bottom flask;
[0072] 2. Place in an ice bath to lower the system temperature to 2.5°C, then add 5 drops of anhydrous pyridine dropwise;
[0073] 3. Heat up to 115°C with stirring, and reflux for 3.5 hours;
[0074] 4. After cooling at room temperature, a large amount of solids appeared, suction filtered, and the filter residue was washed with water and absolute ethanol in sequence, and dried to obtain the crude product of acetylisoferulic acid;
[0075] 5. The crude product is eluted with 300-400 mesh silica gel, and the elution condition is petroleum ether: ethyl acetate: methanol: acetic acid = 300:100:50:10 to obtain acetyl isoferulic acid monomer.
[0076] b) Preparation of 4',7-diacetylisoferulic acid genistein:
[0077] 1. Weigh 0.3g of genistein, 0.1g of acetylisoferulic acid...
Embodiment 3
[0097] Preparation of 4′,7-diacetylisoferulic acid genistein:
[0098] a) Preparation of acetyl isoferulic acid:
[0099] 1. Add 1g isoferulic acid and 20mL acetyl chloride into a 100mL round bottom flask;
[0100] 2. Place in an ice bath to lower the temperature of the system to 5°C, then add 5 drops of anhydrous pyridine dropwise;
[0101] 3. Heat up to 120°C under stirring, and reflux for 4 hours;
[0102] 4. After cooling at room temperature, a large amount of solids appeared, suction filtered, and the filter residue was washed with water and absolute ethanol in sequence, and dried to obtain the crude product of acetylisoferulic acid;
[0103] 5. The crude product is eluted with 300-400 mesh silica gel, and the elution condition is petroleum ether: ethyl acetate: methanol: acetic acid = 300:100:50:10 to obtain acetyl isoferulic acid monomer.
[0104] b) Preparation of 4′,7-diacetylisoferulic acid genistein:
[0105] 1. Weigh 0.3g of genistein, 0.1g of acetylisoferulic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com