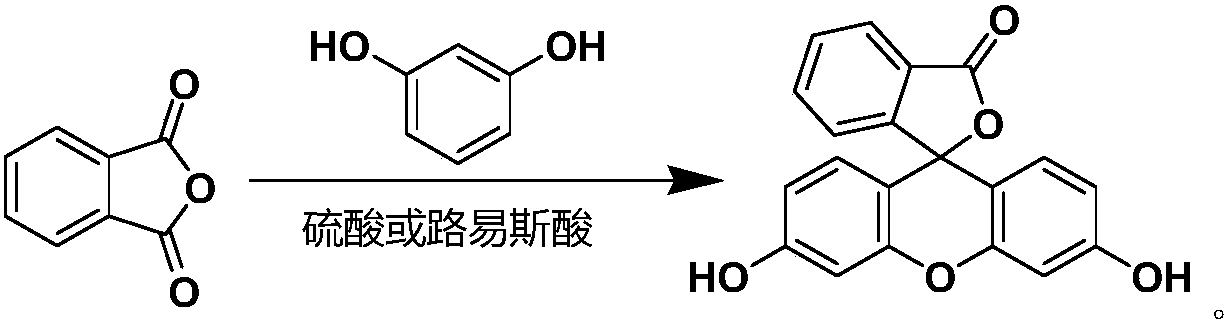
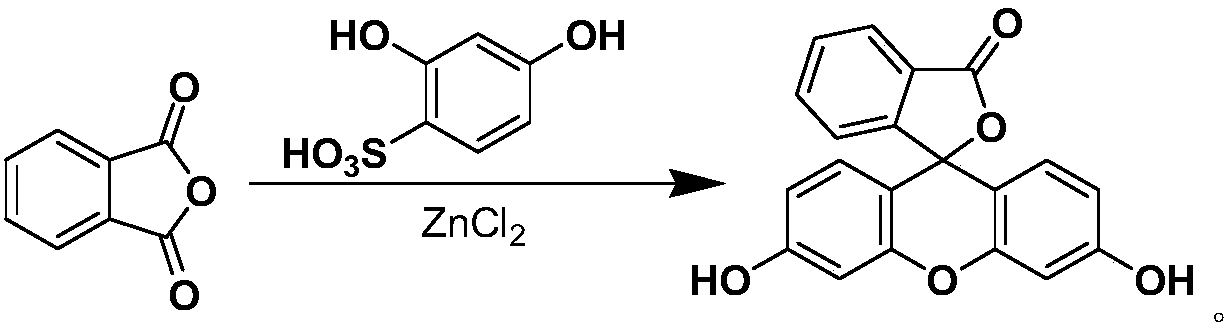
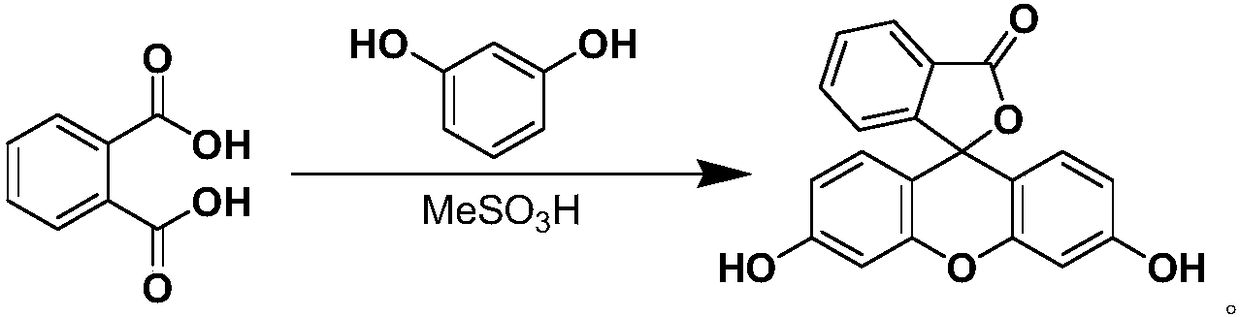
A process for preparing fluorescein
A process method and fluorescein technology, applied in the field of fluorescein preparation, can solve the problems of being difficult to meet industrialized large-scale production, do not meet the requirements of green synthesis, and the catalyst is dangerous, and achieve high production and operation safety, high yield and applicable scope. wide effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0037] Under stirring at room temperature, 30 L of toluene, 3 Kg of phthalic anhydride, 4.4 Kg of resorcinol and 200 g of citric acid were successively added to a 50 L reactor equipped with a water separator.
[0038] Heat to reflux and stir the reaction, and divide water while heating. After insulated reaction for 3 hours, use TLC spot plate to confirm that the reaction of the raw materials is complete and all of them are converted into products, and then change the water separation device to a distillation device. Atmospheric pressure distillation toluene, stop distillation after steaming about 15L toluene.
[0039] The reaction kettle was naturally cooled and crystallized overnight, filtered, and the filter cake was collected. The filter cake was beaten with 1L of pure water for 30 minutes, filtered again, and the filter cake was collected. The filter cake was vacuum-dried at 100° C. for 4 hours to obtain 6.3 kg of an orange-yellow solid with an HPLC purity of 99.5% and a...
example 2
[0042] Under stirring at room temperature, 25L of 2-methyltetrahydrofuran, 3Kg of phthalic anhydride, 4.7Kg of resorcinol and 300g of citric acid were successively added into the 50L reactor.
[0043] After heating to reflux and stirring for 3 hours, use thin-layer chromatography (TLC) spot plate to confirm that the raw materials are completely reacted and all of them are converted into products, and then a distillation device is installed. The 2-methyltetrahydrofuran was distilled at atmospheric pressure, and the distillation was stopped after about 14L of 2-methyltetrahydrofuran was distilled off.
[0044] The reaction kettle was naturally cooled and crystallized overnight, filtered, and the filter cake was collected. The filter cake was beaten with 1L of pure water for 30 minutes, filtered again, and the filter cake was collected. The filter cake was vacuum-dried at 90° C. for 5 hours to obtain 5.7 kg of an orange-yellow solid with an HPLC purity of 99.1% and a yield of 85...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com