Hyperuricemia cell model, building method thereof and application of model to uric acid reduction efficacy evaluation
A hyperuricemia and cell model technology, applied in the field of cell model construction, can solve the problems of long cycle and cumbersome drug screening experiments, and achieve the effect of short cycle and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
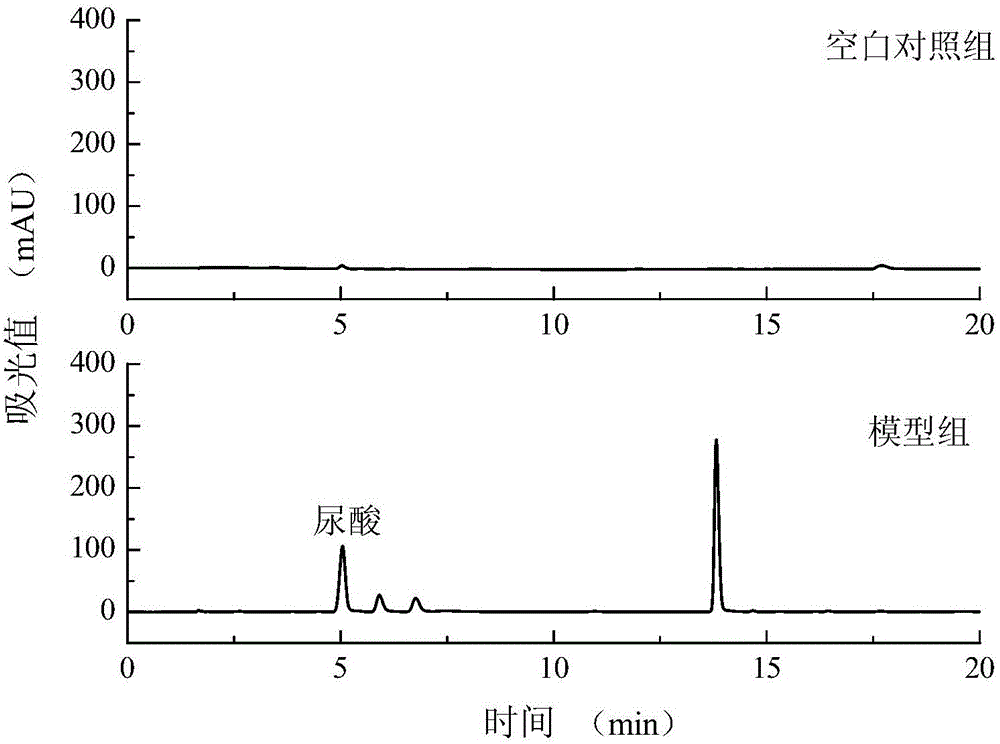
[0037] Construction of a hyperuricemia cell model: the experiment set up a blank control group and a model group.
[0038] Model group: HK-2 cells in the logarithmic growth phase were taken, digested with trypsin, blown with a pipette to make a suspension, and the cell concentration was adjusted to 10 by dilution. 5cells / mL, inoculated in a 12-well plate, cultured in a cell culture incubator for 12 h, discarded the cell supernatant, washed twice with PBS, added 0.25 mmol / L adenosine and incubated for 12 h, each well Add 0.1 IU xanthine oxidase and continue to incubate for 12 hours to obtain a hyperuricemia cell model;
[0039] Blank control group: no adenosine inducer was added, and other conditions were the same as the model group.
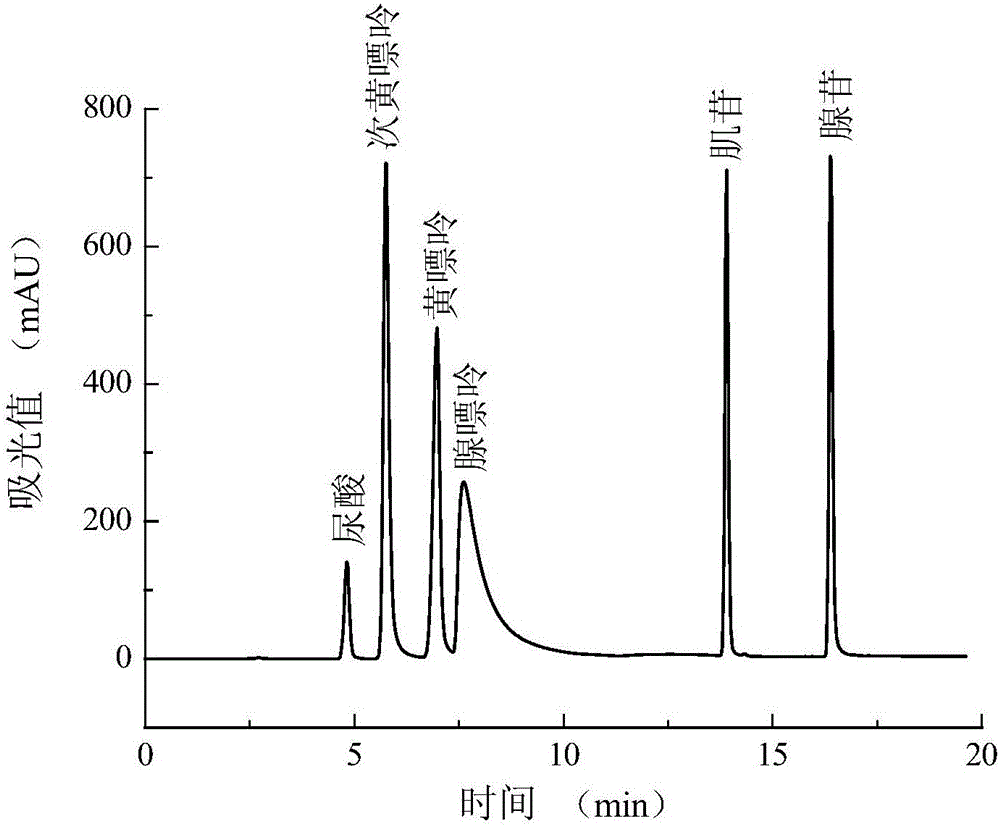
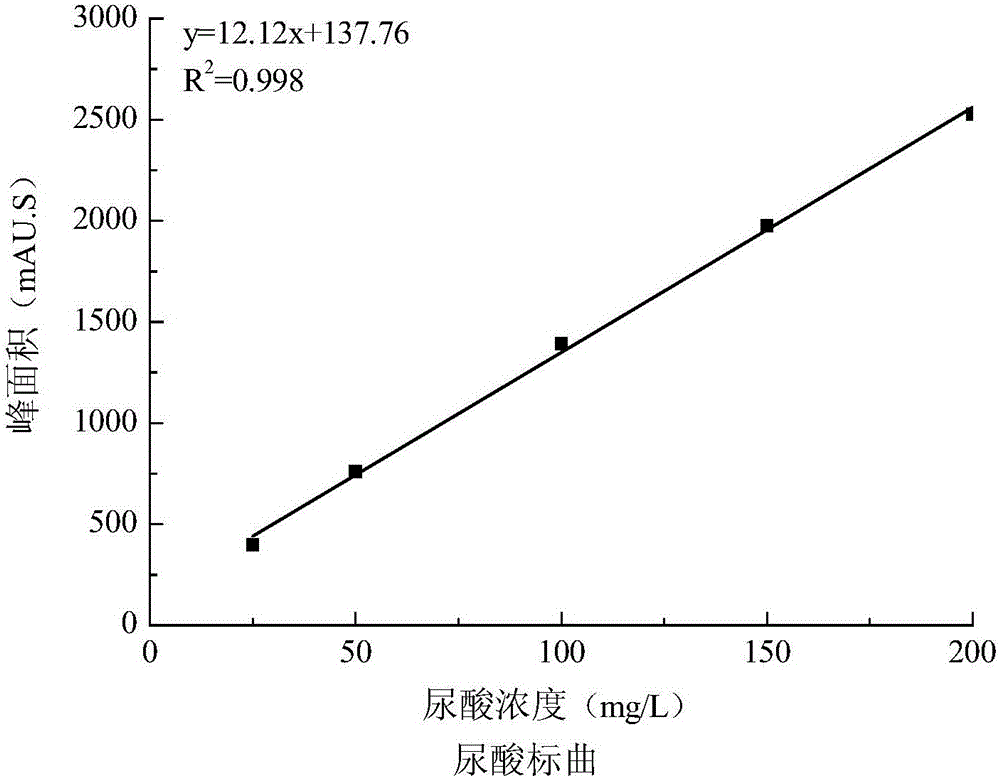
[0040] Take out the cell supernatant, analyze the cell supernatant by HPLC, the high performance liquid chromatogram and the uric acid content figure are respectively as follows image 3 and Figure 4 shown. Depend on image 3 It can be seen...
Embodiment 2
[0042] Construction of a hyperuricemia cell model:
[0043] Take the HK-2 cells in the logarithmic growth phase, add trypsin to digest them, blow them with a pipette to make a suspension, and adjust the cell concentration to 3×10 by diluting 5 cell / mL, inoculated in a 12-well plate, cultured in a cell culture incubator for 12 h, discarded the cell supernatant, washed twice with PBS, added 0.5 mmol / L adenosine and incubated for 12 h, each well Add 0.1 IU xanthine oxidase and continue to incubate for 12 hours to obtain a hyperuricemia cell model; take out the cell supernatant, and analyze the uric acid content in the cell supernatant by HPLC. The results are as follows: Figure 4 shown.
Embodiment 3
[0045] Construction of a hyperuricemia cell model:
[0046] Take the HK-2 cells in the logarithmic growth phase, add trypsin to digest them, blow them with a pipette to make a suspension, and adjust the cell concentration to 3×10 by diluting 5 cell / mL, inoculated in a 12-well plate, cultured in a cell culture incubator for 24 h, discarded the cell supernatant, washed 3 times with PBS, added 0.5 mmol / L adenosine and incubated for 24 h, each well Add 0.2 IU xanthine oxidase and continue to incubate for 24 hours to obtain a hyperuricemia cell model; take out the cell supernatant, and analyze the uric acid content in the cell supernatant by HPLC. The results are as follows: Figure 4 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com