Antibody coating process and myohemoglobin determination reagent kit made with same
A myoglobin and antibody technology, which is applied in biological testing, measuring devices, material inspection products, etc., can solve the problems of high cost and complex preparation process of antibody microspheres, and achieve the effect of improving efficiency, remarkable effect and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] An antibody coating process, the specific process is as follows:
[0031] Step 1, the microspheres and the activator are dissolved in the buffer solution of pH6.0 respectively; the buffer solution in this step adopts potassium dihydrogen phosphate-sodium hydroxide buffer solution, and the microspheres adopt polystyrene latex microspheres , the activator is EDAC.
[0032] Step 2, using an activator to activate the carboxyl groups on the surface of the microspheres; that is, adding the buffer solution containing EDAC to the dissolved microspheres to activate the hydroxyl groups on the surface of the microspheres, and the activation time is 30 minutes.
[0033] Step 3, then adding the antibody to the activated microspheres for coupling, the coupling time is 1h;
[0034] Step 4, adding potassium dihydrogen phosphate-sodium hydroxide buffer solution with pH 8.0 to the antibody-coupled microspheres to continue the reaction, the reaction time is 1 h;
[0035] Step 5. Add cas...
Embodiment 2
[0038] This embodiment is a comparative example of embodiment 1. The difference between this embodiment and embodiment 1 is that this embodiment has modified the specific operation process after step 3. At the same time, the pH value in the whole process of this embodiment is different. The specific process after step three is as follows:
[0039] Step 3, then add the antibody to the activated microspheres for coupling, and the coupling time is 2 hours;
[0040] Step 4: Add casein and glycine to complete the blocking after the reaction is completed, and the blocking time is 30 minutes; after the blocking is completed, add potassium dihydrogen phosphate-sodium hydroxide buffer solution with the same pH value as in step 1, and adjust the pH value to 7.5, Then add trehalose and xylitol as protective agents and store it.
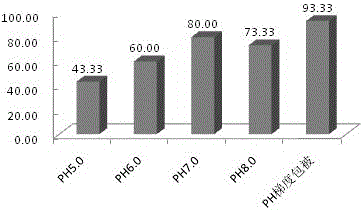
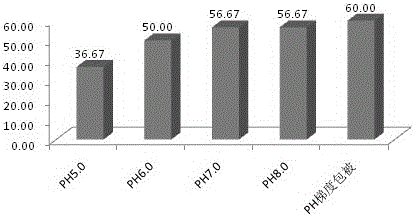
[0041] In this embodiment, buffer solutions with different pH values were used for experiments, and the values of the calibration curve after coating with ...
Embodiment 3
[0046] The difference between this example and Example 1 and Example 2 is that the activators used in this example are selected as EDAC and Sulfo-NHS. Activation, other operation steps are exactly the same as embodiment 1 and embodiment 2.
[0047] This example also gives the values of the calibration curve after coating by the two activation coating methods in Example 1 and Example 2. The values are shown in Table 1, and the coupling under different pH conditions is detected. Efficiency graphs such as figure 2 shown.
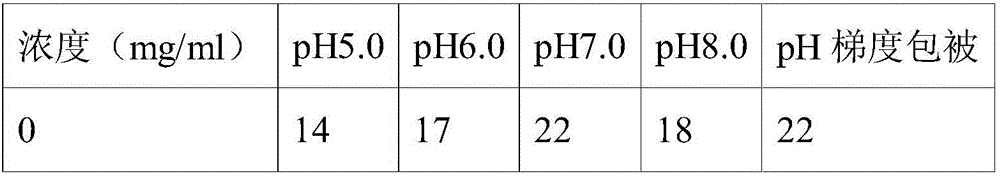
[0048] Table 2
[0049]
[0050]
[0051] pass figure 2 The results show that the coupling efficiency between the antibody and the microspheres is improved to a certain extent by using the pH gradient coating method of the present invention relative to the fixed pH value. But by comparing the test data of embodiment 2 and embodiment 3, it can be seen that when only one activator is used, its coupling efficiency is significantly higher than when ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com