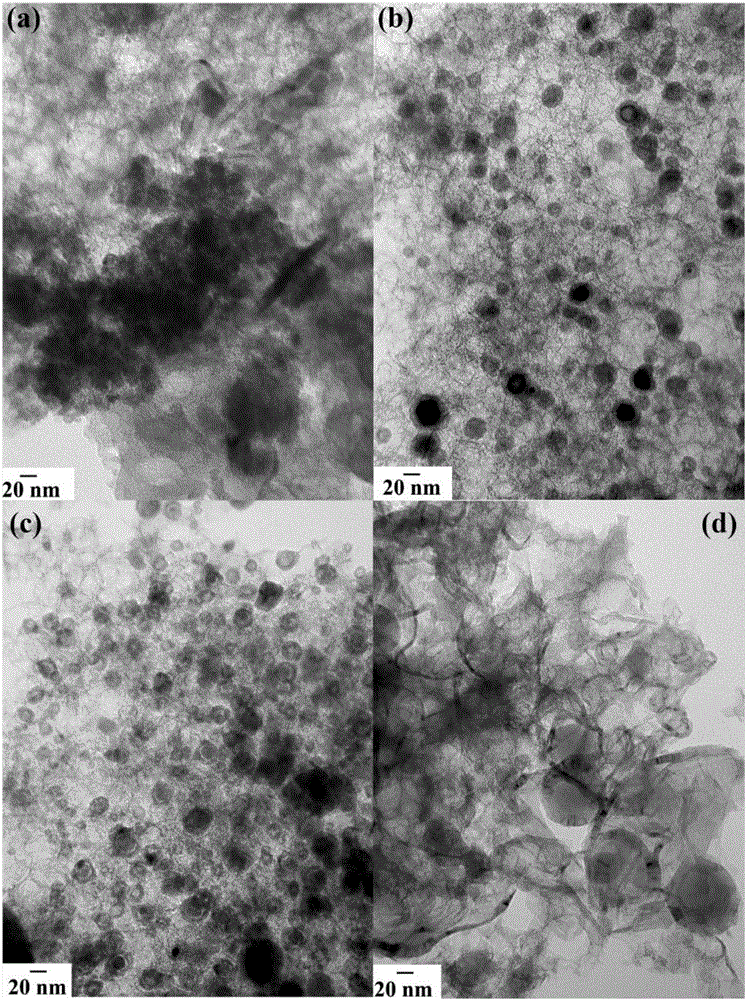
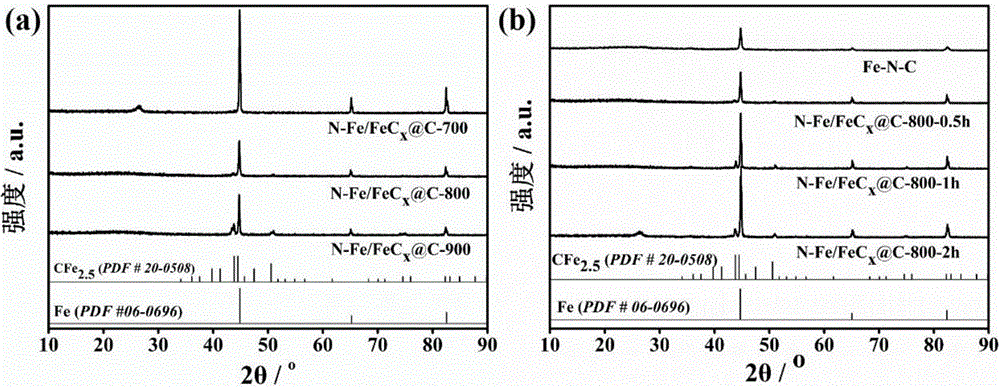
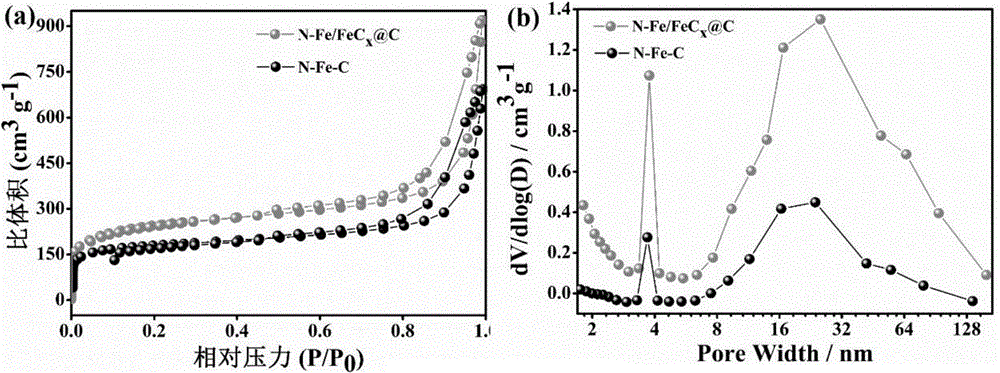
FeCx@NC core-shell structured catalyst and preparation method therefor
A core-shell structure and catalyst technology, applied in structural parts, electrical components, battery electrodes, etc., can solve the problems of difficult control of chemical vapor polymerization process conditions, difficult separation of nanofibers, low yield, etc., and is conducive to large-scale production. , The effect of low cost of raw materials and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: A 1 Fe 1 -G 3 -900(A 1 Fe 3 Refers to the molar mass ratio of aniline and ferric chloride in the raw material is 1:1, G 3 means that the quality of glucose is three times that of aniline, and 900 means that the pyrolysis temperature is 900°C)
[0040] Take 1.8216g of glucose and add it to a certain amount of dilute hydrochloric acid solution, ultrasonically disperse evenly, then take 0.6mL aniline and add it to the hydrochloric acid solution of glucose to ultrasonically disperse evenly, then add 7.5g SiO 2 The sol solution was stirred evenly; then the reaction was placed in an ice-water bath, and 5 mL of 1.2 mol L was added dropwise while vigorously stirring -1 Ferric chloride solution was added to the above mixed solution, and after continuous stirring for 16 hours, it was dried in an air atmosphere at 100° C. for 12 hours to obtain a precursor composite material; 2 At 5°C min under atmosphere -1 The rate is programmed to heat up to 900°C, and the rea...
Embodiment 2
[0042] Example 2: A 1 Fe 5 -G 3 -900(A 1 Fe 5 Refers to the molar mass ratio of aniline and ferric chloride in the raw material is 1:5, G 3 means that the quality of glucose is three times that of aniline, and 900 means that the pyrolysis temperature is 900°C)
[0043] Take 1.8216g of glucose and add it to a certain amount of dilute hydrochloric acid solution, ultrasonically disperse evenly, then take 0.6mL aniline and add it to the hydrochloric acid solution of glucose to ultrasonically disperse evenly, then add 7.5g SiO 2 The sol solution was stirred evenly; then the reaction was placed in an ice-water bath, and 25 mL of 1.2 mol L was added dropwise while vigorously stirring -1 Ferric chloride solution was added to the above mixed solution, and after continuous stirring for 16 hours, it was dried in an air atmosphere at 100° C. for 12 hours to obtain a precursor composite material; 2 At 5°C min under atmosphere -1 The rate is programmed to heat up to 900°C, and the re...
Embodiment 3
[0045] Example 3: A 1 Fe 6 -G 3 -900(A 1 Fe 6 Refers to the molar mass ratio of aniline and ferric chloride in the raw material is 1:6, G 3 means that the quality of glucose is three times that of aniline, and 900 means that the pyrolysis temperature is 900°C)
[0046] Take 1.8216g of glucose and add it to a certain amount of dilute hydrochloric acid solution, ultrasonically disperse evenly, then take 0.6mL aniline and add it to the hydrochloric acid solution of glucose to ultrasonically disperse evenly, then add 7.5g SiO 2 The sol solution was stirred evenly; then the reaction was placed in an ice-water bath, and 30 mL of 1.2 mol L was added dropwise while vigorously stirring -1 Ferric chloride solution was added to the above mixed solution, and after continuous stirring for 16 hours, it was dried in an air atmosphere at 100° C. for 12 hours to obtain a precursor composite material; 2 At 5°C min under atmosphere -1 The rate is programmed to heat up to 900°C, and the re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com