Preparation method for 1,2,4,5-tetra-substituted imidazole derivatives and catalyst for preparation
A technology of imidazole derivatives and four substitutions, which is applied in the field of ionic liquid catalysis, can solve the problems of complex product purification process, low raw material utilization rate, and difficult biodegradation, so as to reduce side reactions and impurities, improve utilization rate, and Good biodegradability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
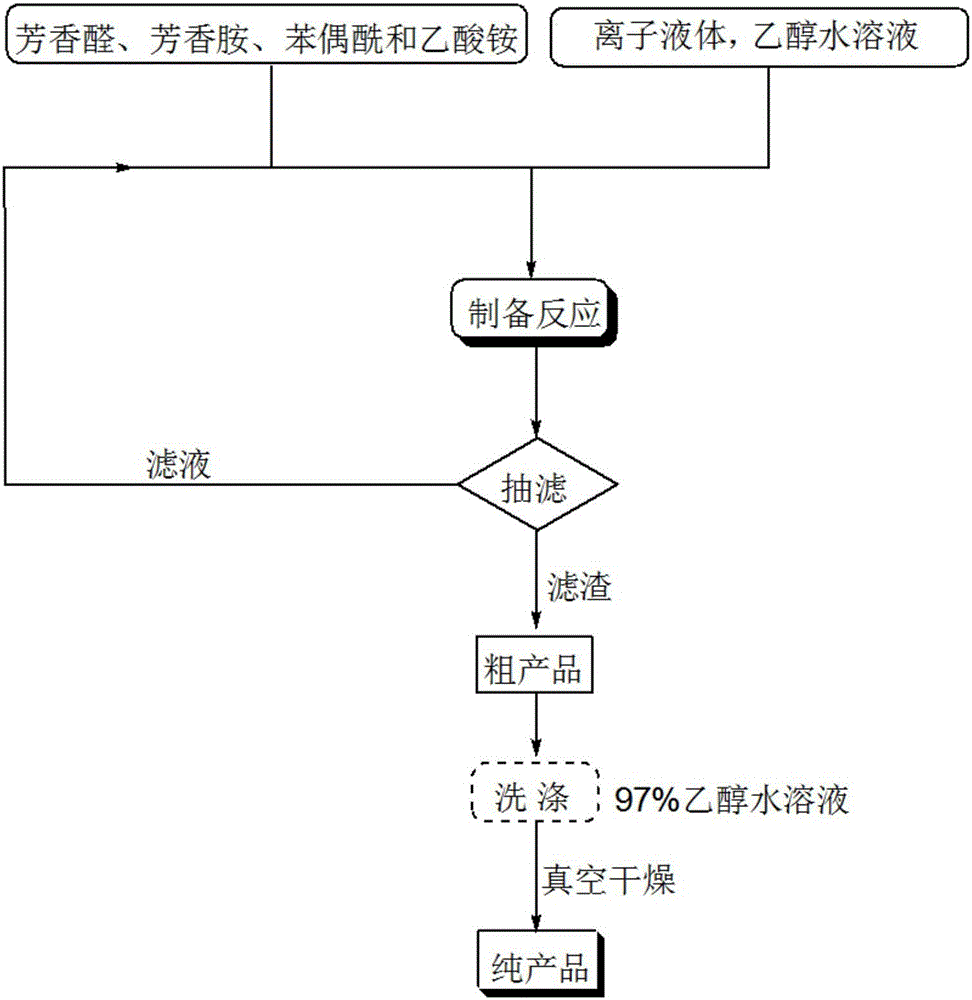
[0042] The concrete steps of the preparation method of 1,2,4,5-tetrasubstituted imidazole derivatives of the present invention are:
[0043] (1) Weighing of raw materials: Accurately weigh the reaction raw materials benzil, aromatic aldehyde, aromatic amine and ammonium acetate according to the molar ratio of 1:1:1:(1-1.2).
[0044] (2) Catalyzed preparation: add the weighed reaction raw materials into the ethanol aqueous solution respectively, after fully dissolving and mixing uniformly, continue to add the acidic ionic liquid catalyst with benzil molar amount of 7-12% therein, and carry out under stirring conditions Heating to reflux reaction to obtain a solid precipitate, the volume of the above-mentioned aqueous ethanol solution in milliliters is 6 to 8 times the molar amount of benzil in millimoles, and the volume percent concentration of ethanol contained in the aqueous ethanol solution is 95 to 97 %, and the reaction pressure of the reflux reaction is an atmospheric pre...
Embodiment 1
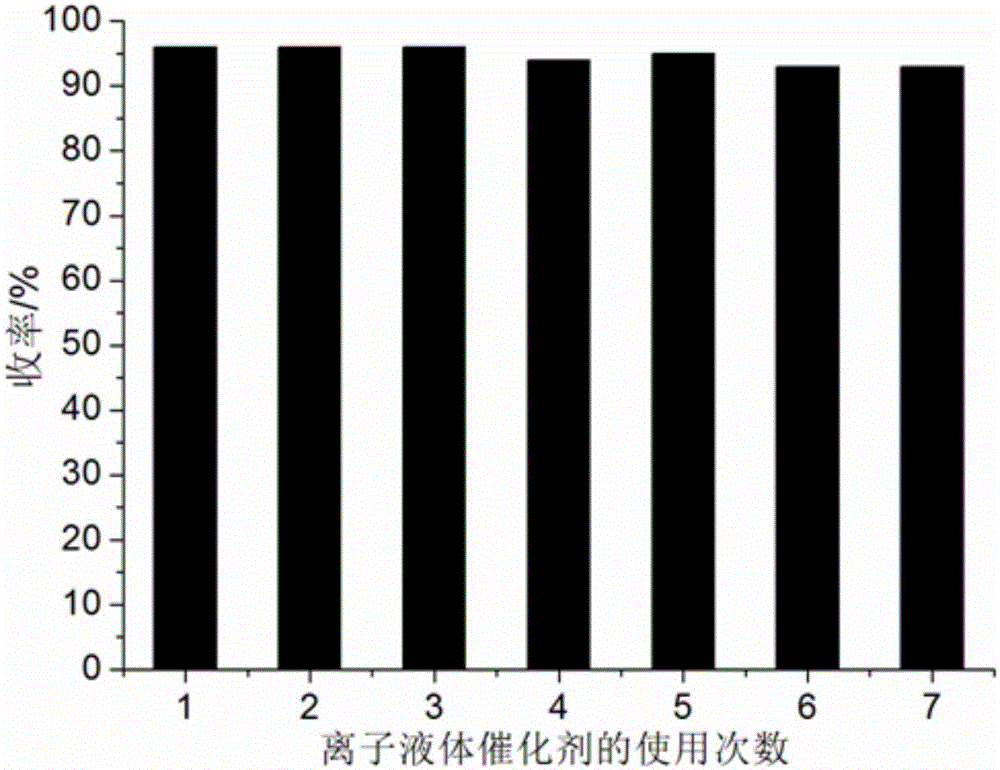
[0052] 1mmol benzil, 1mmol p-chlorobenzaldehyde, 1mmol p-chloroaniline, 1mmol ammonium acetate and 0.08mmol ionic liquid catalyst were added to 8ml 97% ethanol aqueous solution in a 50ml single-necked bottle with a stirring bar and a condenser. Heat to reflux for 15 minutes, TLC (thin plate chromatography) detection, the raw material point disappears, cool to room temperature, crush the precipitated solid, let it stand, filter with suction, wash the filter residue with 97% ethanol aqueous solution, and vacuum dry to obtain 1,2-bis (4-Chlorophenyl)-4,5-diphenyl-1H-imidazole, the yield is 96%, directly add benzil, p-chlorobenzaldehyde, p-chloroaniline and ammonium acetate in the collected filtrate and repeat use.
[0053] 1,2-bis(4-chlorophenyl)-4,5-diphenyl-1H-imidazole obtained in this example: m.p.187~189°C; IR(KBr):1597, 1495, 1409cm -1 ; 1 H NMR (300MHz, DMSO-d 6 ): δ=6.89~7.65 (m, 18H, ArH)
Embodiment 2
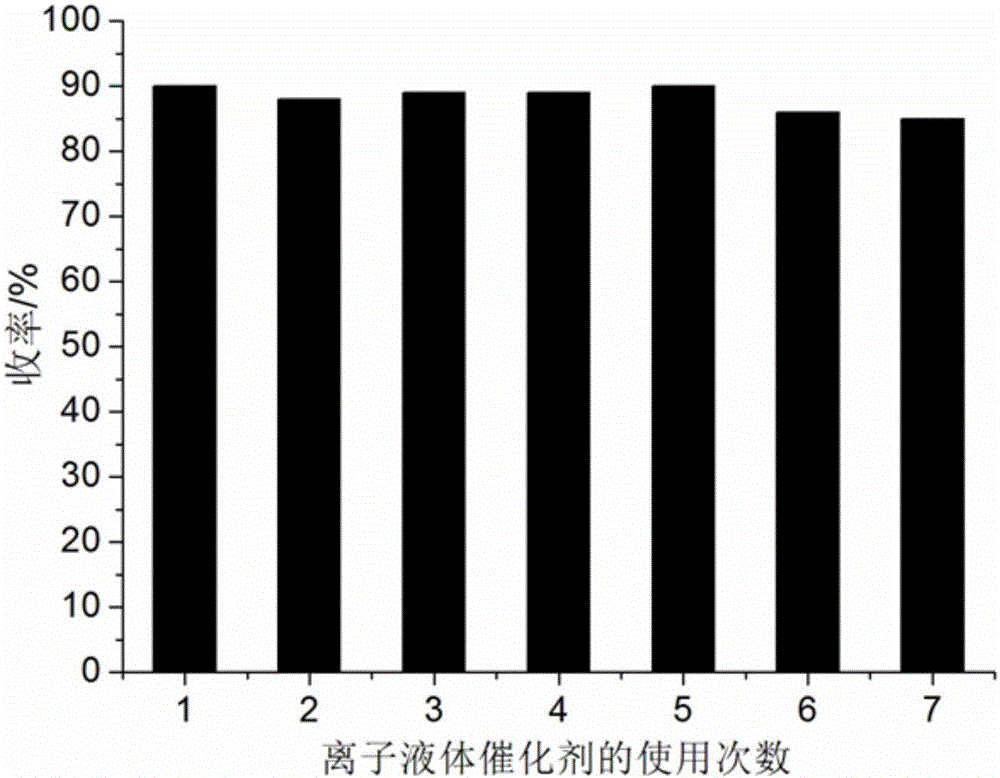
[0055] 1mmol benzil, 1mmol benzaldehyde, 1mmol benzylamine, 1.2mmol ammonium acetate and 0.10mmol ionic liquid were respectively added to 6ml 97% ethanol aqueous solution in a 50ml single-necked bottle with a stirring bar and a condenser tube. Heat to reflux for 32 minutes, TLC (thin plate chromatography) detection, the raw material point disappears, cool to room temperature, crush the precipitated solid, let stand, filter with suction, the filter residue is washed with 97% ethanol aqueous solution, and vacuum dried to obtain 1-benzyl -4,5-diphenyl-2-phenyl-1H-imidazole, the yield is 88%. The collected filtrate is directly added with benzil, benzaldehyde, benzylamine and ammonium acetate for repeated use.
[0056] The performance parameters of 1-benzyl-4,5-diphenyl-2-phenyl-1H-imidazole obtained in this example are as follows: m.p.163~165°C; IR (KBr): 1596, 1493, 1471, 1412cm -1 ; 1 H NMR (300MHz, DMSO-d 6 ): δ=5.07(s, 2H, CH 2 ), 6.69-7.60 (m, 20H, ArH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com