Induced t7 RNA polymerase
A polymerase and induced expression technology, which is applied in the field of T7 RNA polymerase and ribonucleic acid polymerase to induce and control gene expression, can solve the problems of no tissue specificity, poor resolution of chemical inducers, and inability to achieve uniform induction. The method is simple and achieves the effect of gene expression level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1 Light-induced protein-protein interaction regulates T7 RNAP activity ( Figure 3a )
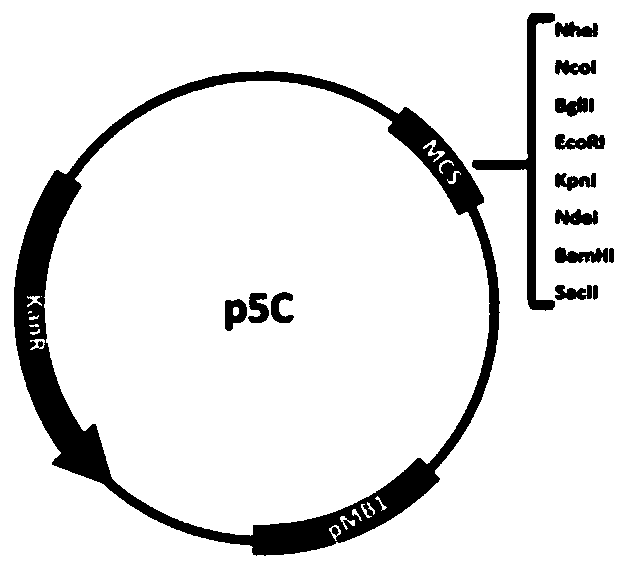
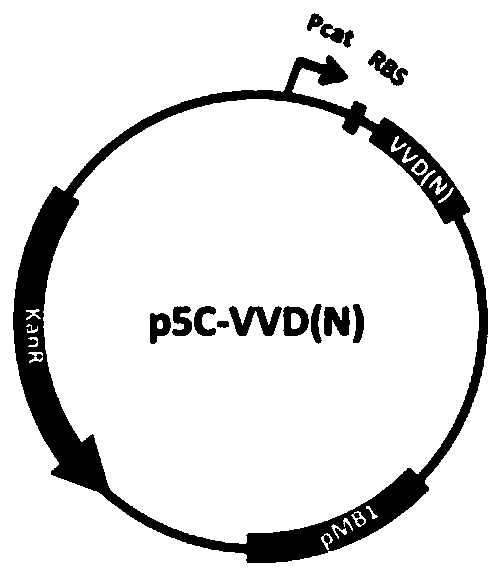
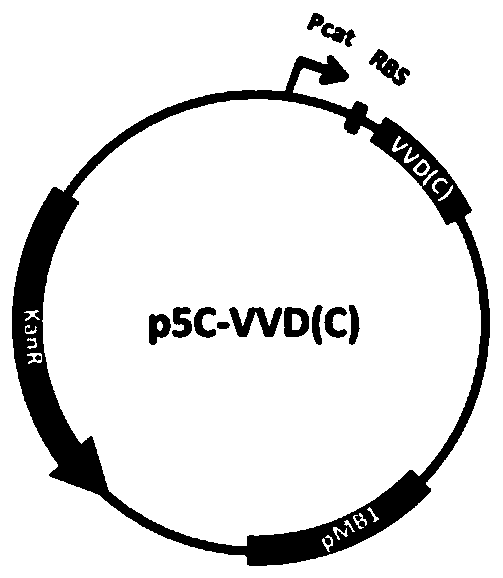
[0055] PaT7P-1, an induction system designed in the present invention to recover T7 RNA polymerase (coding sequence is SEQ ID NO.5, corresponding amino acid sequence is SEQ ID NO.30) through light-induced protein-protein interaction. The induction system was obtained by the following method: using the plasmid J61002 (BioBrick code BBa_J61002) as a template, replacing the ampicillin resistance gene with the kana resistance gene, obtaining the p5C plasmid as a template for the light induction system, and introducing multiple Cloning site (MCS) to obtain p5C-MCS ( Figure 1a ). A constitutive promoter Pcat (BioBrick codeBBa_I14033) was inserted between NheI and NcoI, and RBS (BioBrick code BBa_B0034) was inserted between NcoI / BglII and KpnI / NdeI restriction sites respectively. Gene synthesis of VVD-36 9 Sequence (the N-terminus of light-sensitive protein VVD removes 36 amino...
Embodiment 2
[0057] Example 2 Light-induced allosteric regulation of the self-assembly of the T7 RNAP resolution system ( Figure 3b )
[0058] PaT7P-2 is a light-induced allosteric regulation system designed in the present invention. Light activation promotes the self-assembly of protein fragments to restore the activity of T7 RNA polymerase. The induction system was obtained by the following method: plasmid paT7P-1 was digested with NdeI and SacII, using PrimeSTAR HS DNA polymerase and primer pair C565-F2 (SEQ ID NO.21), C565-R1 (SEQ ID NO.20 ) to perform PCR amplification to obtain the C565-I fragment, and use the recombinase Exnase II (Vazyme) to recombine the C565-I fragment to the restriction position, then use PrimeSTAR MAX DNA polymerase and primer pair pMag-F (SEQ IDNO. 22) and pMag-R (SEQ ID NO.23) for site-directed mutation PCR, and then converted and sequenced after DpnI digestion to obtain paT7P-2 ( Figure 1f )(Note: pMag is a mutant of VVD, its nucleic acid sequence and am...
Embodiment 3
[0059] Example 3 Light-induced allosteric regulation of Full-length T7 RNAP activity ( Figure 3c )
[0060] PaT7P-3, light-induced allosteric regulation of T7 RNA polymerase, is a simplified version of paT7P-2 described in Example 2. The induction system is obtained by the following method: paT7P-1 is double digested with EcoRI and BamHI, and the VVD coding sequence is removed. Using pT7P-2 as a template, use PrimeSTAR HS DNA polymerase and primers to perform PCR amplification on pMag1-F (SEQ ID NO.24) and pMag1-R (SEQ ID NO.25) to obtain pMag1 fragment (containing linker1 sequence) , using the recombinase ExnaseMultis (Vazyme) to recombine pMag1 and linker2 into the above-mentioned EcoRI / BamHI restriction sites to obtain paT7P-3 ( Figure 1g ). To detect the light-induced properties of paT7P-3, the pJ61-R reporter system mentioned in Example 1 was still selected, and paT7P-3 and pJ61-R were co-transformed into E.coli, single clones were selected, liquid cultured overnight...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com