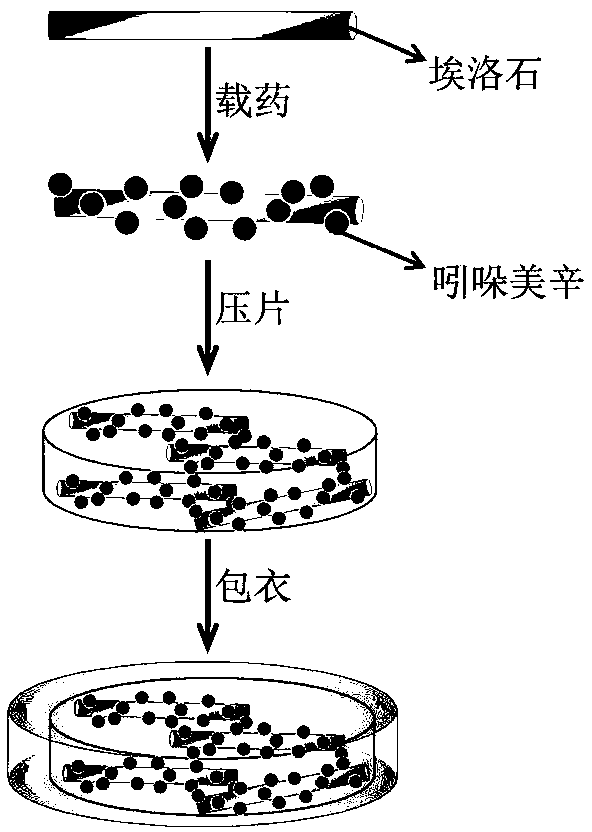
Enteric composite drug-loading system with halloysite nanotube as skeleton and preparation method thereof
A technology of halloysite nanotubes and skeletons, which is used in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. The problems of small strength and high preparation cost can achieve the effect of improving drug loading rate and improving slow-release enteric solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
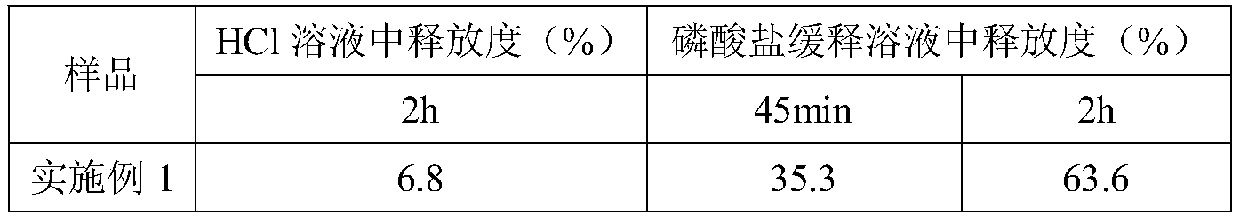
[0017] (1) Weigh 4g of acetone, 0.025g of indomethacin, and 0.25g of halloysite nanotubes respectively, then add indomethacin into the acetone, stir and mix to form solution A, and slowly drop solution A with a syringe into 0.25g of halloysite nanotubes, stirred evenly at 30°C, dried in a vacuum oven at 50°C, and then placed the dried powder in a tablet press to form tablets with a diameter of 1 cm and a thickness of 0.15 cm. The tablet weighs 0.275g, and each tablet contains 0.025g of indomethacin;
[0018] (2) Weigh 0.5g of hypromellose phthalate, 0.1g of soluble starch, and 0.003g of polyvinylpyrrolidone (Woke, PVP-K30, GR 100g), and add them to 15ml of absolute ethanol and 4ml of In the mixed solution of deionized water, stir for 30 minutes to prepare solution B;
[0019] (3) Use the dipping-pulling method to slowly and uniformly immerse the tablet prepared in step (1) into the solution B of step (2) cooled to 0°C, and slowly take it out after 5 seconds of composite cross...
Embodiment 2
[0021] (1) Weigh 2g of acetone, 0.030g of indomethacin, and 0.35g of halloysite nanotubes respectively, then add indomethacin into the acetone, stir and mix to form solution A, and slowly drop solution A with a syringe into 0.35g of halloysite nanotubes, stirred evenly at 37°C, dried in a vacuum oven at 40°C, and then placed the dry powder in a tablet press to be pressed into tablets with a diameter of 1 cm and a thickness of 0.22 cm. The weight of the tablet is 0.38g, and each tablet contains 0.030g of indomethacin;
[0022] (2) Weigh 0.6g hypromellose phthalate, 0.2g soluble starch, 0.002g polyvinylpyrrolidone (Wokai, PVP-K30, GR 100g), add to 20ml absolute ethanol and 5ml In the mixed solution of deionized water, stir for 60 minutes to prepare solution B;
[0023] (3) Use the dipping-pulling method to slowly and uniformly immerse the tablet prepared in step (1) into the solution B of step (2) cooled to 0°C, and slowly take it out after 3 seconds of composite crosslinking, ...
Embodiment 3
[0025] (1) Weigh 4g of acetone, 0.040g of indomethacin and 0.30g of halloysite nanotubes respectively, then add indomethacin into the acetone, stir and mix to form solution A, and slowly drop solution A with a syringe into 0.30 g of halloysite nanotubes, stirred evenly at 40°C, placed in a vacuum oven at 50°C, and dried, and then the dried powder was placed in a tablet press to be pressed into tablets with a diameter of 1 cm and a thickness of 0.18 cm. The tablet weighs 0.34g, and each tablet contains 0.040g of indomethacin;
[0026] (2) Weigh 0.4g hypromellose phthalate, 0.3g soluble starch, 0.01g polyvinylpyrrolidone (Wokai, PVP-K30, GR 100g), add to 30ml absolute ethanol and 5ml In the mixed solution of deionized water, stir for 60 minutes to prepare solution B;
[0027] (3) Use the dipping-pulling method to slowly and uniformly immerse the tablet prepared in step (1) into the solution B of step (2) cooled to 0°C, and slowly take it out after 3 seconds of composite crossli...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| release amount | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com