Preparation method and products of non-noble metal catalyst
A non-precious metal and catalyst technology, applied in nanomaterial preparation technology and electrochemical fields, can solve problems such as low catalytic activity, and achieve the effects of simple preparation method, aerobic reduction and oxygen evolution activity, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation method of embodiment 1 is specifically as follows:
[0033] (1) Cobalt nitrate and copper nitrate are respectively dissolved in anhydrous methanol according to a molar ratio of 4:1, mixed and stirred evenly to obtain a uniformly dissolved first solution;
[0034] (2) Dissolve the mixture of cobalt nitrate and copper nitrate and the organic ligand in anhydrous methanol at a molar ratio of 1:4 to obtain a second solution that is uniformly dissolved; in Example 1, the organic ligand is dimethylimidazole ;
[0035] (3) uniformly mix the above first solution and the second solution, and after standing at room temperature for 24 hours, centrifuge or suction filter it to obtain a precipitate, and dry the precipitate at 80° C. to obtain a catalyst precursor;
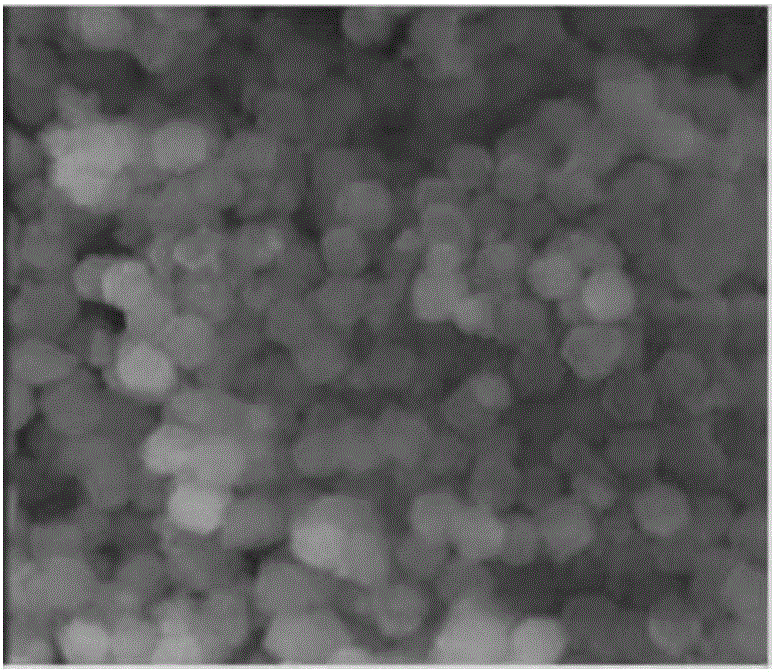
[0036] figure 1 Shown is the scanning photograph of the catalyst precursor that obtains in the preparation process of embodiment 1; figure 1 The size of the particles in the medium is between 200nm and ...
Embodiment 2
[0047] The preparation method of embodiment 2 is specifically as follows:
[0048] (1) Cobalt nitrate and copper nitrate are respectively dissolved in anhydrous methanol according to a molar ratio of 1:1, mixed and stirred evenly to obtain a uniformly dissolved first solution;
[0049] (2) Dissolve the mixture of cobalt nitrate and copper nitrate and dimethylimidazole in anhydrous methanol according to the molar ratio of 1:4 to obtain a second solution that is uniformly dissolved; wherein the mixing ratio of cobalt nitrate and copper nitrate is 1 :1;
[0050] (3) uniformly mix the first solution and the second solution above, let the mixed solution stand at room temperature for 24 hours, then perform centrifugation or suction filtration to obtain a precipitate, and dry the precipitate at 80° C. to obtain a catalyst precursor;
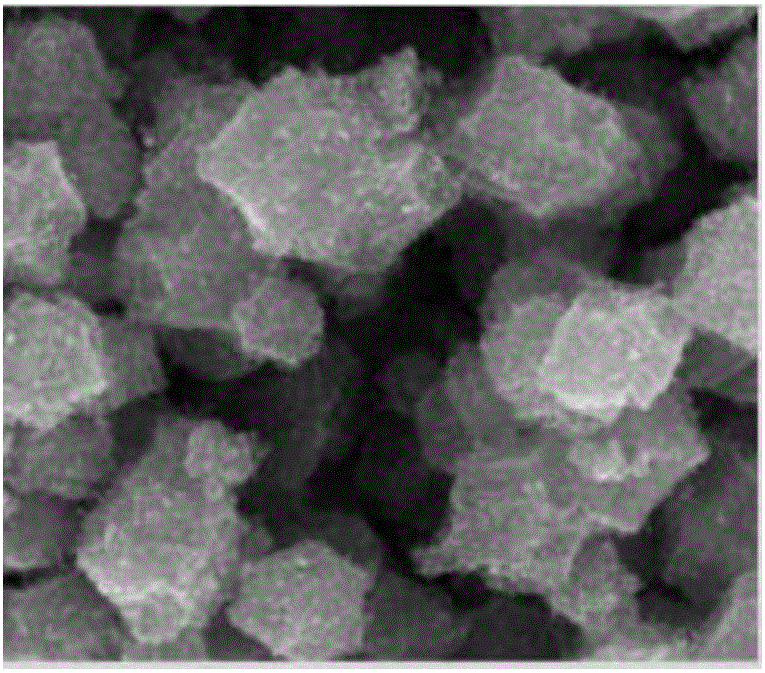
[0051] (4) in N 2 In the atmosphere, at a temperature of 900°C, the catalyst precursor was pyrolyzed at a heating rate of 10°C / min for 5 hours to obt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com