Synthetic method of N-(1H,1H,2H,2H-perfluorinated octyl) acrylamide
A perfluorooctyl and acrylamide technology, which is applied in the field of preparation of fluorine-containing compounds, can solve the problems of sensitive heating conditions, explosion, and unsuitability for industrial production, and achieve the effects of easy industrialization, simple process, and avoiding the possibility of explosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
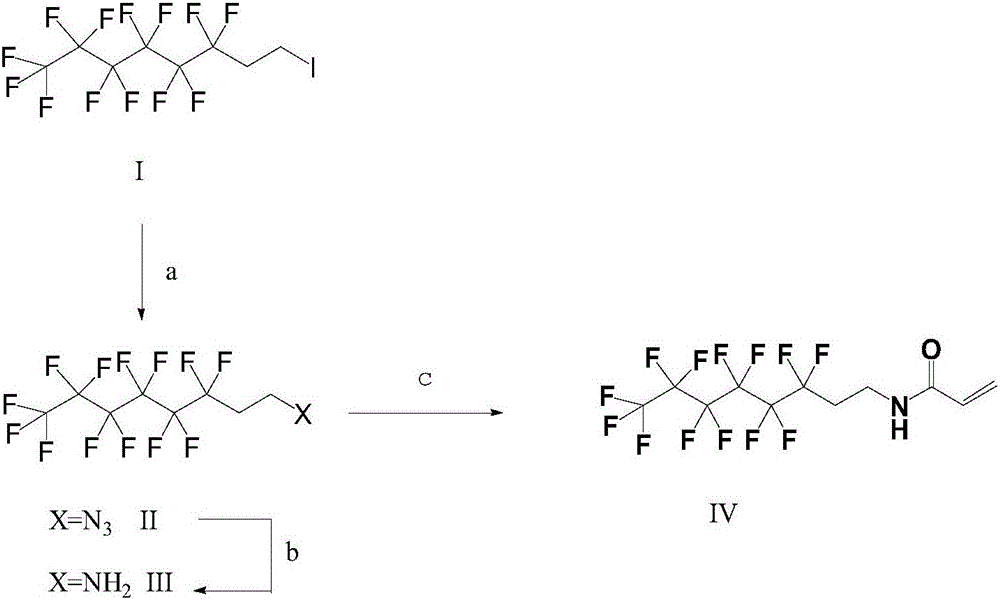
[0023] A kind of synthetic method of N-(1H, 1H, 2H, 2H-perfluorooctyl) acrylamide comprises the following steps:
[0024] (1) Synthesis of 1H, 1H, 2H, 2H-perfluorooctyl azide
[0025] In a 20-liter reactor, add 1500 g (3.16 mol) of 1H, 1H, 2H, 2H-perfluorohexylethyl iodide, 3 L of dimethylformamide, and 230 g (3.5 mol) of sodium azide. The mixture was stirred at room temperature for 3 h, GC showed complete conversion, selectivity 99%, added 3 L of water, allowed to stand, separated layers, the aqueous layer was washed three times with methyl tert-butyl ether, and the three-time washed organic layer was washed three times with brine to obtain Crude 1H,1H,2H,2H-perfluorooctyl azide containing methyl tert-butyl ether.
[0026] (2) Synthesis of 1H, 1H, 2H, 2H-perfluorooctylamine
[0027] Take 442 grams of 1H, 1H, 2H, 2H-perfluorooctyl azide prepared by the method of step (1) and place it in an autoclave, and replace the air with hydrogen. 40 grams of Pd / C with a Pd mass percent...
Embodiment 2
[0031] (1) Synthesis of 1H, 1H, 2H, 2H-perfluorooctyl azide
[0032] In a 20-liter reactor, add 1500 g (3.16 mol) of 1H, 1H, 2H, 2H-perfluorohexylethyl iodide, 2 L of dimethyl sulfoxide, and 205.4 g (3.16 mol) of sodium azide. The mixture was stirred at room temperature for 5 h, GC showed complete conversion, selectivity 99%, added 6 L of water, allowed to stand, separated layers, the aqueous layer was washed three times with methyl tert-butyl ether, and the three-time washed organic layer was washed three times with brine to obtain Crude 1H,1H,2H,2H-perfluorooctyl azide containing methyl tert-butyl ether.
[0033] (2) Synthesis of 1H, 1H, 2H, 2H-perfluorooctylamine
[0034] Take 442 grams of 1H, 1H, 2H, 2H-perfluorooctyl azide prepared by the method of step (1) and place it in an autoclave, and replace the air with hydrogen. 40 grams of Pd / C with a Pd mass percentage of 10% was added, and hydrogen gas was introduced to carry out a hydrogenation reduction reaction at 2 MPa. ...
Embodiment 3
[0038] (1) Synthesis of 1H, 1H, 2H, 2H-perfluorooctyl azide
[0039] In a 5-liter reactor, add 375 g (0.79 mol) of 1H, 1H, 2H, 2H-perfluorohexylethyl iodide, 1 L of N-methylpyrrolidone, and 77 g (1.18 mol) of sodium azide. The mixture was stirred at room temperature for 7 h, GC showed complete conversion, selectivity 99%, added 1 L of water, allowed to stand, separated layers, the aqueous layer was washed three times with methyl tert-butyl ether, and the organic layer washed three times with brine three times to obtain Crude 1H,1H,2H,2H-perfluorooctyl azide containing methyl tert-butyl ether.
[0040] (2) Synthesis of 1H, 1H, 2H, 2H-perfluorooctylamine
[0041] Take 221 grams of 1H, 1H, 2H, 2H-perfluorooctyl azide prepared by the method of step (1) and place it in an autoclave, and replace the air with hydrogen. 40 grams of Pd / C with a Pd mass percentage of 8% was added, and hydrogen gas was introduced to carry out a hydrogenation reduction reaction at 1.5 MPa. The reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com