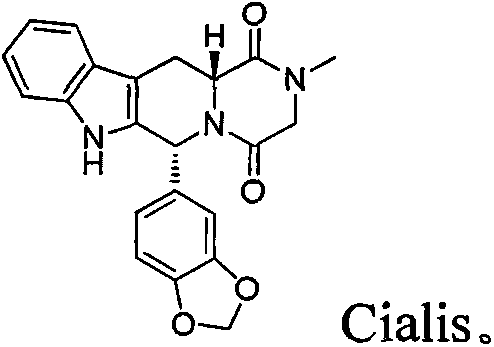
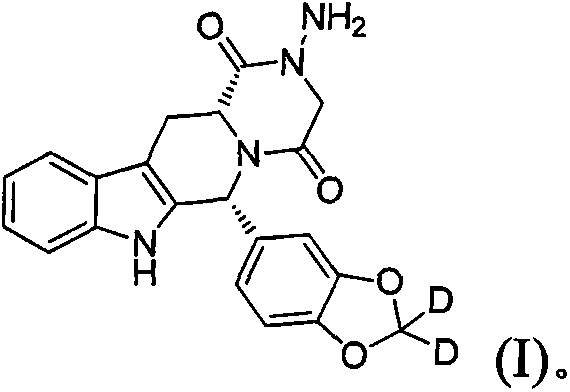
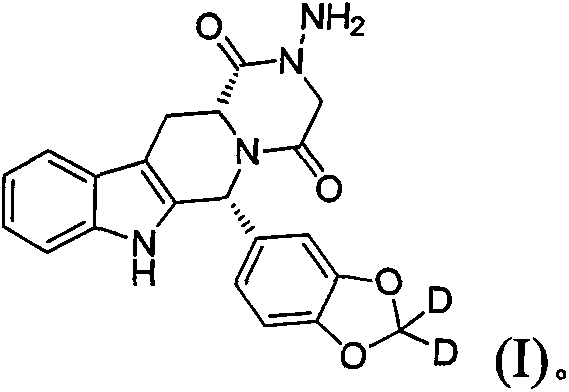
Type 5 phosphodiesterase inhibitors and their applications
A technology of phosphodiesterase and use, which is applied in the field of medicine, can solve problems such as half-life differences, and achieve good inhibitory effects and good pharmacokinetic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] The preparation of the compound represented by embodiment 1 formula (I) (number: DDCI01)
[0065] step 1:
[0066]
[0067] Table A
[0068]
[0069] Into a 500 mL reaction vial equipped with a reflux condenser was charged the reaction reagents and solvents shown in Table A. in N 2 In the atmosphere, the reaction was refluxed at 120°C for 6h. The reaction was monitored for completion by thin layer chromatography (TLC). After cooling the reaction solution to room temperature, the Cs was removed by filtration 2 CO 3 solid, with CH 2 Cl 2 Wash the filter cake. The filtrate was concentrated to dryness under reduced pressure, and the resulting residue was washed with CH 2 Cl 2 dissolved, followed by washing once with water and once with saturated NaCl solution, respectively. Anhydrous MgSO for organic phase 4 Dry, filter, and concentrate to dryness under reduced pressure to obtain a crude product. The obtained crude product was purified by silica gel colum...
Embodiment 2
[0087] Example 2: Inhibition of PED5
[0088] Method: Sildenafil (S1431, selleck) was used as positive control to calibrate, and homogeneous time energy transfer (Homogenous Time Resolve Fluoresce, HTF) method (Cat. ) The dose-effect relationship of inhibitory ability measured the half inhibitory concentration IC of the sample 50 .
[0089] sample IC 50 value
Cialis 111.6nM
[0090] DDCI01 33.6nM DDCI02 注
566.2nM
[0091] NOTE: The structure of compound DDCI02 is shown below:
[0092]
Embodiment 3
[0093] Embodiment 3: pharmacokinetic research
[0094] experimental method:
[0095] Stock solution preparation: Weigh 1.13 mg of Cialis, 1.15 mg of DDCI02 and 1.23 mg of DDCI01, and dissolve them in 1.13, 1.15, and 1.23 ml of dimethyl sulfoxide respectively to prepare a stock solution with a concentration of 1.00 mg / ml.
[0096] Sample preparation method: one-step protein precipitation method
[0097] Precipitating agent: Acetonitrile (with 5.00 ng / ml of verapamil)
[0098] Standard curve and QC sample preparation: Transfer 5.00 μl of standard curve or QC working solution and 45.0 μl of blank plasma to a 1.5 ml centrifuge tube. The final concentrations of the standard curve samples were 1.00, 2.50, 5.00, 10.0, 50.0, 100, 500, and 1000 ng / mL, respectively. The concentrations of quality control samples were 2.50, 5.00, 50.0 and 800 ng / ml, respectively. Dilute quality control samples to a concentration of 5000 ng / ml.
[0099] Plasma sample preparation: transfer 100 microlit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com