Efficient CIK cell preparation and detection method
A cell and efficient technology, applied in biochemical equipment and methods, animal cells, vertebrate cells, etc., can solve the problems of low CD3+CD56+ double positive expression rate, unstable preparation process, and low proliferation multiple of CIK cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
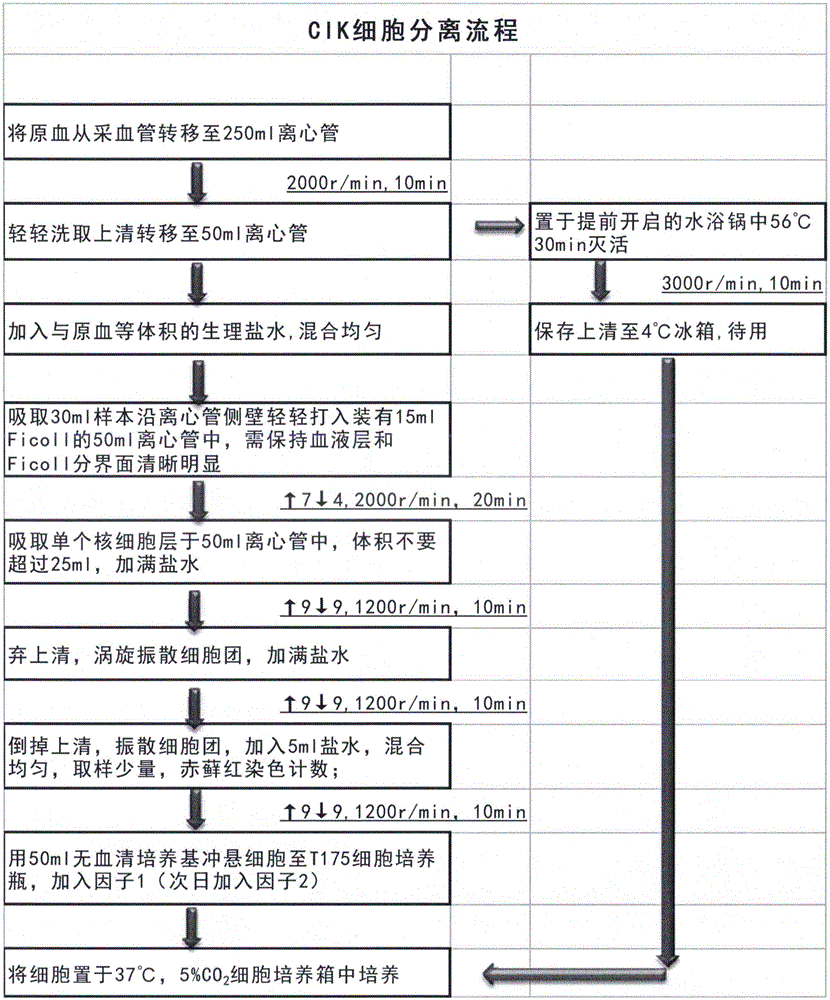
[0099] The method for preparing high-efficiency CIK cells of the present invention comprises the following steps:
[0100] (1) Whole blood test and blood sample storage: Use a pipette to transfer the original blood from the blood collection tube to a centrifuge tube, blow and beat repeatedly to mix well: take out the blood sample for microbial detection, and store the blood sample in a cryopreservation tube for refrigerated storage. As a file for subsequent inspection;
[0101] (2) Plasma treatment: Put the centrifuge tube containing the original blood into the centrifuge, balance and centrifuge, transfer the upper layer plasma to the centrifuge tube, close the lid tightly, stick the sealing film, and place it in a 56°C water bath to inactivate , after inactivation, the pellet was discarded by centrifuge, and the supernatant FicoLL was saved for later use;
[0102] (3) Separation of mononuclear cells: add an equal volume of normal saline to the original blood, pipette to dilu...
Embodiment 2
[0203] Embodiment 2, the difference between the high-efficiency CIK cell preparation and detection method of the present invention and the above-mentioned embodiment 1 is only: 4.1 In the detection of cell number and activity: the number of cells requires 3-4×10 9; 4.2 Cell sterility test: observe the result after 48 hours, if the culture medium is still clear, the result is negative. 4.5 Cell phenotype detection: Wash once with normal saline.
[0204] Embodiment 2, the method for preparing and detecting high-efficiency CIK cells of the present invention differs from the above-mentioned embodiment 1 only in that: 4.1 In the detection of cell number and activity: the number of cells requires 5-6×10 9 ; 4.2 Cell sterility test: observe the result after 72 hours, if the culture medium is still clear, it means the result is negative. Wash with normal saline twice.
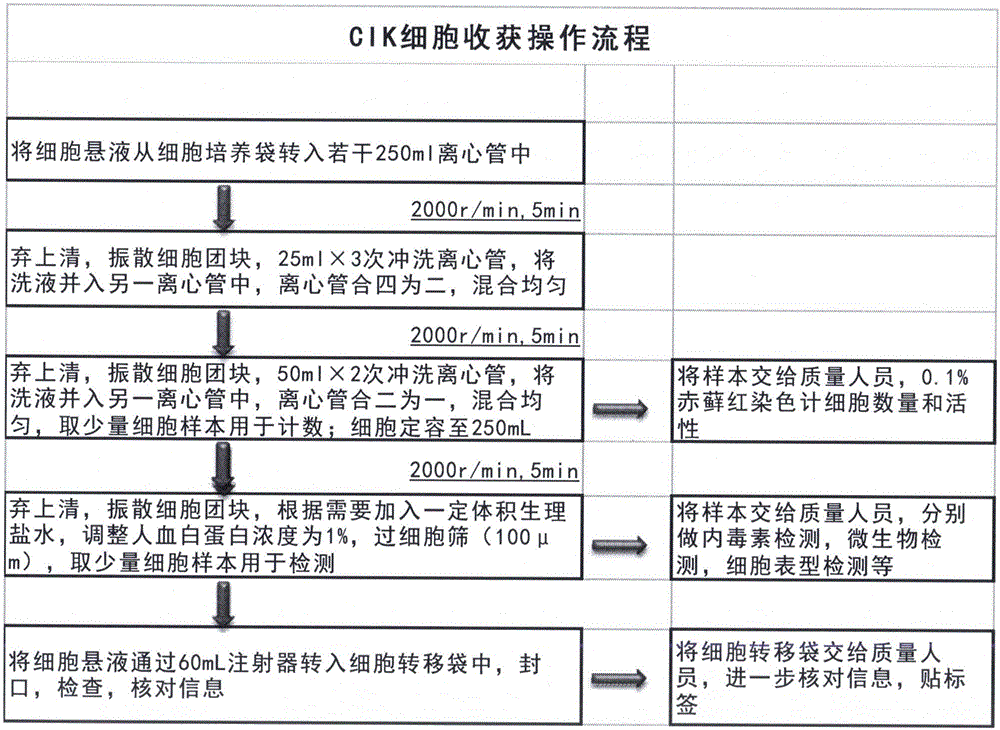
[0205] For the above examples, please refer to the attached CIK cell preparation flow chart.
[0206] The present...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com