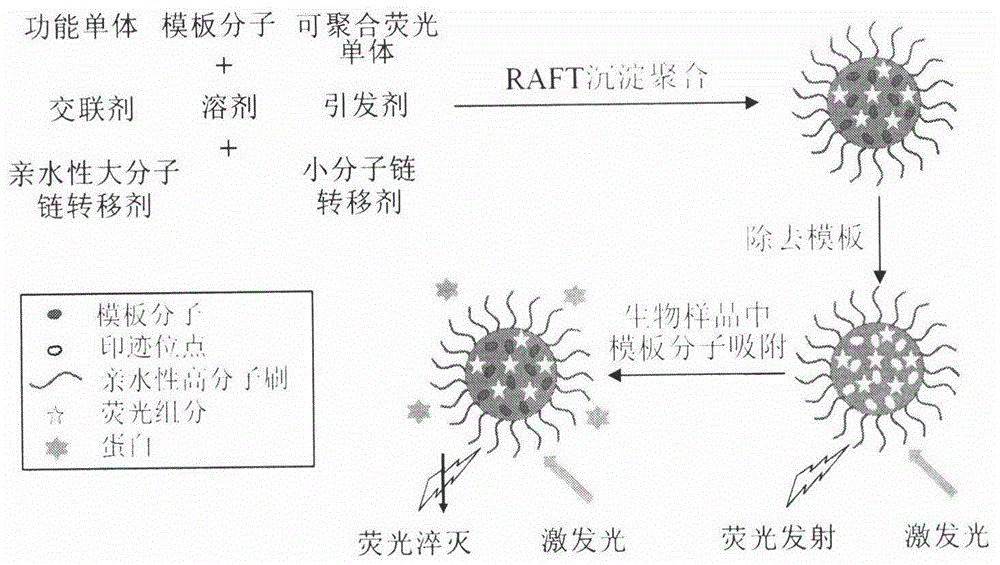
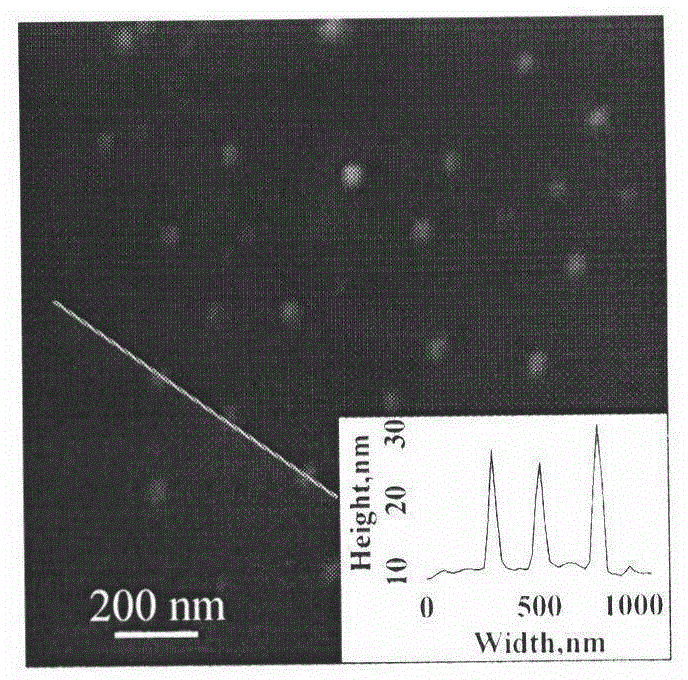
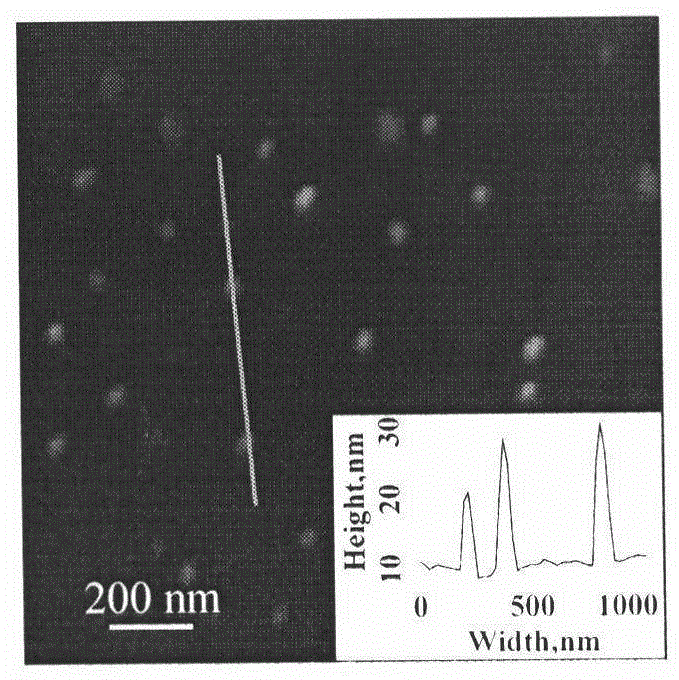
Molecularly imprinted polymer sensing material suitable for biological samples and preparation method of sensing material
A molecularly imprinted, polymer technology, applied in material excitation analysis, fluorescence/phosphorescence, etc., can solve the problems of time-consuming and limit the practical application of molecularly imprinted polymers.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0035] Add 0.834mmol of tetracycline Tc into a 100mL round-bottomed flask filled with 60mL of acetonitrile / dimethylformamide (4:1, volume / volume), stir it with a magnet to dissolve it completely, then add 0.834mmol of methacrylic acid, 2.501mmol Ethylene glycol dimethacrylate, 0.0834mmol polymerizable fluorescent monomer (2-hydroxyethyl methacrylate) 9-anthracene ester, 0.0552mmol small molecule chain transfer agent cumyl dithiobenzoate (CDB), 0.0342mmol poly-2-hydroxyethyl methacrylate macromolecular chain transfer agent (M n,NMR =5140) and 0.0566 mmol of azobisisobutyronitrile (AIBN). After deoxygenating with argon for 30 minutes, the reaction system was sealed, placed in a constant temperature oil bath at 60°C, reacted for 24 hours, and centrifuged to obtain the reaction product.
[0036] The reaction product was washed successively with methanol / acetic acid (9:1, volume / volume) and methanol until the template-free molecules were washed out. After drying, freeze-dry in va...
example 2
[0039] Add 0.834mmol of tetracycline Tc into a 100mL round-bottomed flask filled with 60mL of acetonitrile / dimethylformamide (4:1, volume / volume), stir it with a magnet to dissolve it completely, then add 0.834mmol of methacrylic acid, 2.501mmol Ethylene glycol dimethacrylate, 0.0834mmol polymerizable fluorescent monomer (2-hydroxyethyl methacrylate) 9-anthracene ester, 0.0552mmol small molecule chain transfer agent cumyl dithiobenzoate (CDB), 0.0342mmol poly-2-hydroxyethyl methacrylate macromolecular chain transfer agent (M n,NMR =3610) and 0.0566 mmol of azobisisobutyronitrile (AIBN). After deoxygenating with argon for 30 minutes, the reaction system was sealed, placed in a constant temperature oil bath at 60°C, reacted for 24 hours, and centrifuged to obtain the reaction product.
[0040] The reaction product was washed successively with methanol / acetic acid (9:1, volume / volume) and methanol until the template-free molecules were washed out. After drying, freeze-dry in va...
example 3
[0043] Add 0.834mmol of tetracycline Tc into a 100mL round-bottomed flask filled with 60mL of acetonitrile / dimethylformamide (4:1, volume / volume), stir it with a magnet to dissolve it completely, then add 0.834mmol of methacrylic acid, 2.501mmol Ethylene glycol dimethacrylate, 0.0834mmol polymerizable fluorescent monomer (2-hydroxyethyl methacrylate) 9-anthracene ester, 0.0552mmol small molecule chain transfer agent cumyl dithiobenzoate (CDB), 0.0342mmol poly-2-hydroxyethyl methacrylate macromolecular chain transfer agent (M n,NMR =2610) and 0.0566 mmol of azobisisobutyronitrile (AIBN). After deoxygenating with argon for 30 minutes, the reaction system was sealed, placed in a constant temperature oil bath at 60°C, reacted for 24 hours, and centrifuged to obtain the reaction product.
[0044] The reaction product was washed successively with methanol / acetic acid (9:1, volume / volume) and methanol until the template-free molecules were washed out. After drying, freeze-dry in va...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com