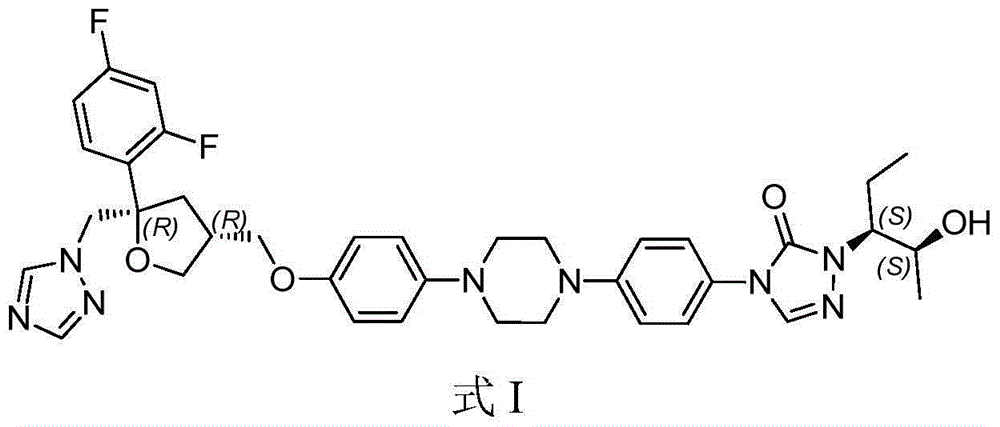
Posaconazole liquid suspension and preparation method thereof
A technology of posaconazole and suspension, which is applied in the field of medicine, can solve the problems of large particle size variation of posaconazole, large fluctuation of batch particle size, complicated preparation process, etc., and achieve good bioavailability, particle size The effect of small change and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1 (using microcrystalline cellulose-carboxymethylcellulose sodium complex CL-611 type as suspending agent to prepare posaconazole liquid suspension)
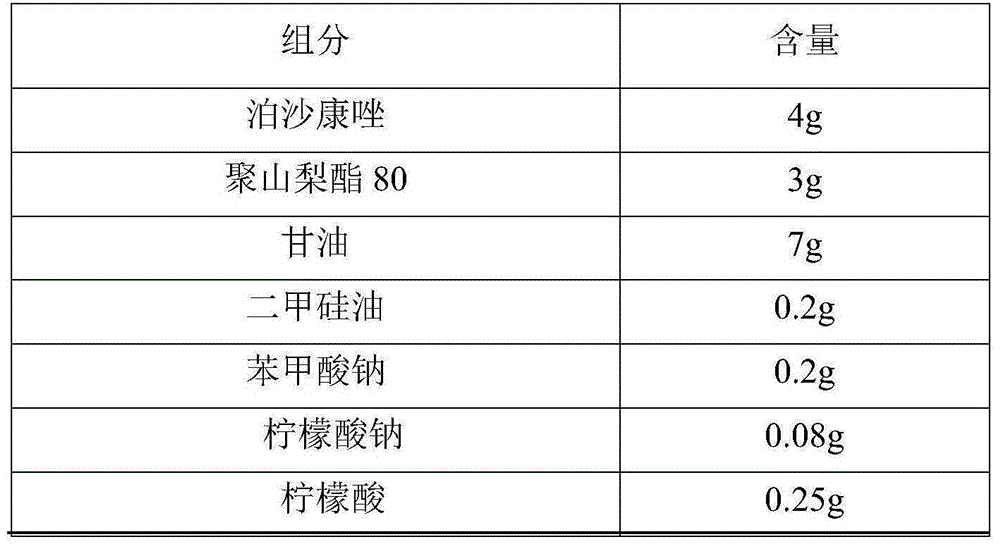
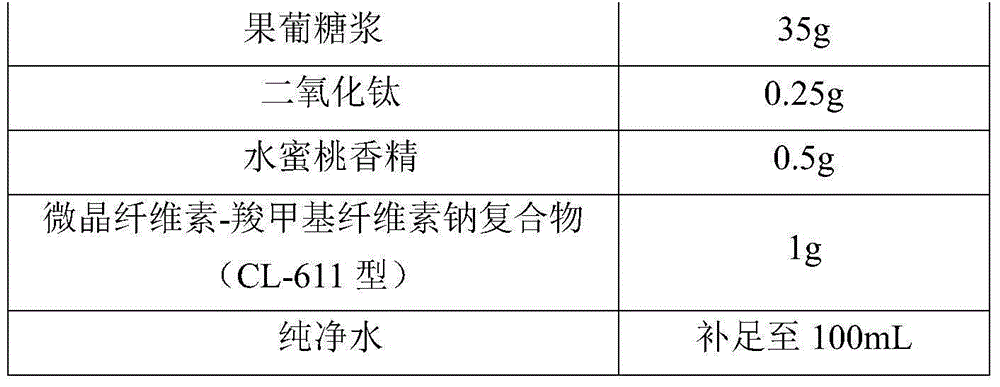
[0038] Taking the total amount of raw materials of the posaconazole liquid suspension as 100mL, the raw material formula of the posaconazole liquid suspension of the present embodiment is as shown in Table 1:
[0039] The raw material formula of the posaconazole liquid suspension of table 1 embodiment 1
[0040]
[0041]
[0042] The preparation method of posaconazole liquid suspension in the present embodiment comprises the steps:
[0043](1) Add simethicone, polysorbate 80, a part of glycerin, sodium benzoate and posaconazole to a part of purified water, stir (stirring speed 300rpm) and mix evenly, and use a high-pressure homogenizer to homogenize (homogeneous pressure 1000bar) to The particle diameter of posaconazole is 2000nm, gets posaconazole concentrated solution; In step (1), the amount of descri...
Embodiment 2
[0046] Embodiment 2 (using microcrystalline cellulose-carboxymethylcellulose sodium complex RC-581 type as suspending agent to prepare posaconazole liquid suspension)
[0047] Taking the total amount of raw materials of the posaconazole liquid suspension as 100mL, the raw material formula of the posaconazole liquid suspension of the present embodiment is as shown in Table 2:
[0048] The raw material formula of the posaconazole liquid suspension of table 2 embodiment 2
[0049]
[0050]
[0051] The preparation method of posaconazole liquid suspension in the present embodiment comprises the steps:
[0052] (1) Add simethicone, polysorbate 80, a part of glycerin, sodium benzoate and posaconazole to a part of purified water, stir (stirring speed 50rpm) and mix well, use a high-pressure homogenizer to homogenize (homogeneous pressure 800bar) to The particle diameter of posaconazole is 1000nm, gets posaconazole concentrated solution; In step (1), the amount of described a p...
Embodiment 3
[0055] Embodiment 3 (using microcrystalline cellulose-carboxymethylcellulose sodium complex RC-591 type as suspending agent to prepare posaconazole liquid suspension)
[0056] Taking the total amount of raw materials of the posaconazole liquid suspension as 100mL, the raw material formula of the posaconazole liquid suspension of the present embodiment is as shown in table 3:
[0057] The raw material formula of the posaconazole liquid suspension of table 3 embodiment 3
[0058]
[0059]
[0060] The preparation method of posaconazole liquid suspension in the present embodiment comprises the steps:
[0061] (1) Add simethicone, nonionic surfactant, part of glycerin, sodium benzoate and posaconazole to a part of purified water, stir (stirring speed 500rpm) and mix evenly, and homogenize with a high-pressure homogenizer (homogeneous pressure 2500bar) The particle diameter until posaconazole is 2000nm, gets posaconazole concentrated solution; In step (1), the amount of desc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com