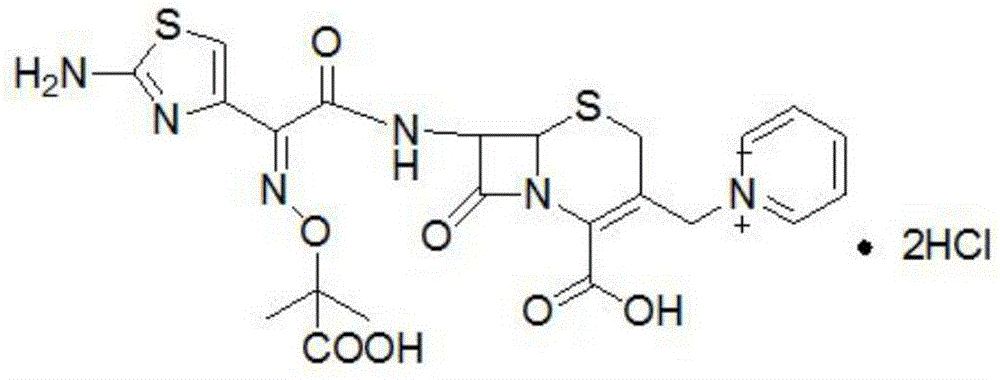
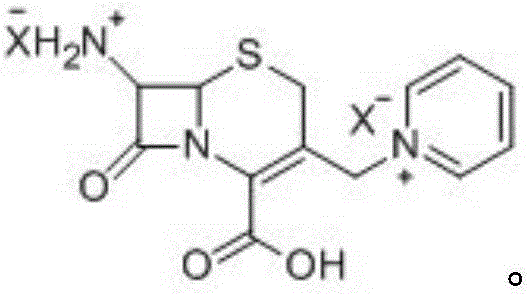
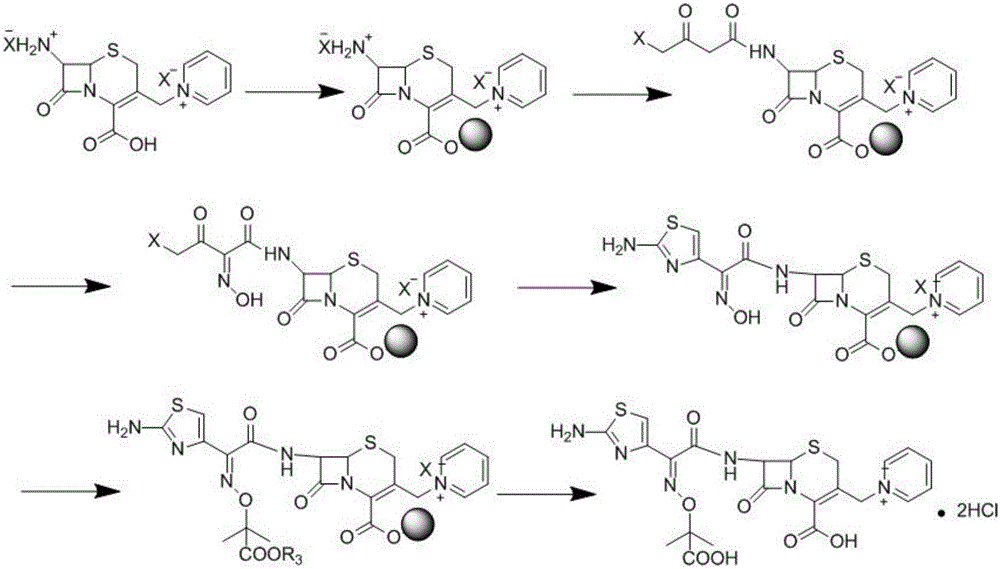
Solid phase synthesis method of ceftazidime hydrochloride
A solid-phase synthesis method, the technology of ceftazidime, applied in the direction of organic chemistry, organic chemistry, etc., can solve the problems of poor purity, cumbersome operation, low total yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0082] Embodiment one: embodiment one: the bridging of 7-APCA and solid phase carrier
[0083]
[0084] In the glazed glass column, 30g 7-APCA is reacted with chloromethyl resin in 100ml DMF, and TLC detects that there is no raw material residue, so that it is fully bridged with the solid phase carrier, and after the reaction is completed, use N2 (argon, air, Gases such as helium) flow to remove solvent, then wash the resin successively with 100ml of dichloromethane and pyridine (or solvents such as chloroform, toluene) respectively, and directly carry out the next step reaction.
[0085] In Embodiment 1, the chloromethyl resin can also be replaced by polystyrene-styrene divinyl cross-linked resin, polyacrylamide, polyethylene-glycol resin, carboxyl resin, amino resin, hydrazide resin, and the like.
[0086] According to the functional group difference of the resin, the dosage ratio of 7-APCA and resin is adjusted. For example: when polystyrene and polyethylene resins are ...
Embodiment 2
[0089] Embodiment two: prepare the ceftazidime tert-butyl ester of solid-phase bridge
[0090]
[0091] A. Add 60g of trimethylalkylacetamide and 200ml of dichloromethane solution into the glazed glass column, cool down to -20°C, and add 78g of 4-bromoacetylacetyl bromide under stirring. After the addition was completed, the reaction was incubated at -10°C for 4 hours, and after the reaction was completed, N 2 (Gas such as argon, air, helium) flow to remove solvent, solvent recovery can be reused, then wash the resin successively with 100ml of dichloromethane and pyridine (or solvents such as chloroform, toluene), and directly carry out the next step reaction.
[0092] In this step, 4-bromoacetylacetyl bromide can also be replaced by e.g.:
[0093]
[0094] X: chlorine, iodine, bromine;
[0095] R1: chlorine, iodine, bromine, methoxy, ethoxy, hydroxyl, hydrogen, amino.
[0096] The amount of the amidating agent (such as: 4-bromoacetylacetyl bromide) is: the molar rati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com