Preparation method of hydroxyl iron phosphate-based battery material with novel morphology
A technology based on iron hydroxyphosphate and battery materials, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems of poor electrochemical stability, uneven size distribution, and uncontrollable crystal morphology of materials, and achieve low cost, No agglomeration phenomenon, the effect of uniform size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
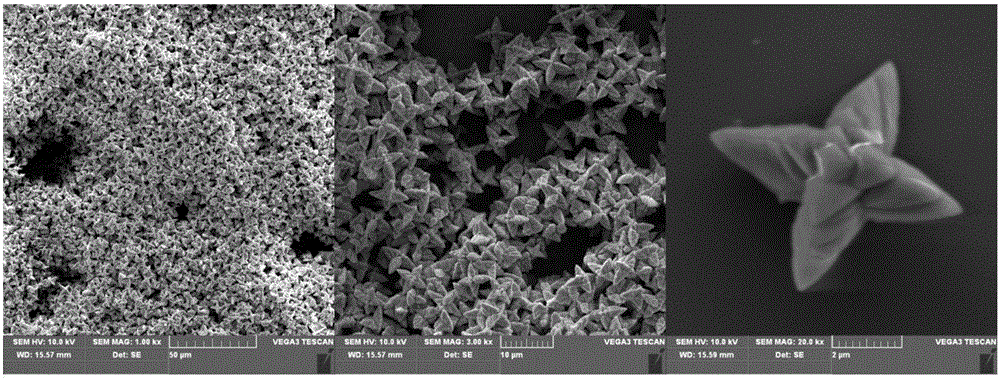
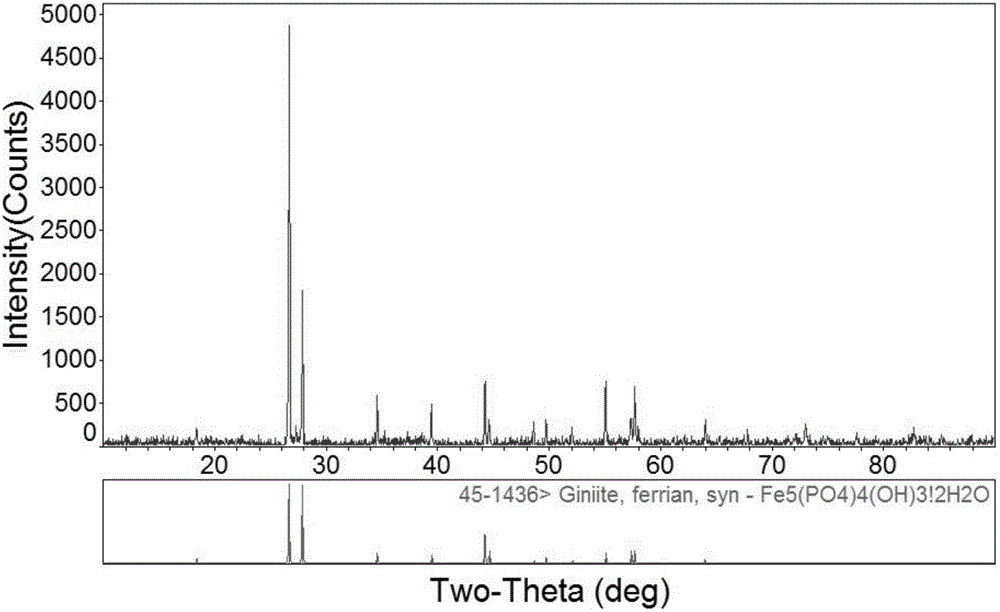
[0027] In this example, FeCl3·6H2O and Na3PO4·12H2O are used as raw materials, and the molar ratio of Fe:P is 1:1 for batching, and the hydrothermal method is used to prepare ferric hydroxyphosphate. The details are as follows: Weigh 5.406g of FeCl3·6H2O and dissolve it in a small amount of deionized water, then add 7.602g of Na3PO4·12H2O, after stirring continuously for 20 minutes, add an appropriate amount of deionized water to make the volume of the entire solution 100ml. The prepared solution was transferred to a polytetrafluoroethylene liner, and heated at 140° C. for 24 hours. Then it was centrifuged, washed and dried at 80°C for 2h. The product obtained after drying was redissolved in 100ml of 1mol / L hydrochloric acid solution, stirred continuously for 30 minutes, centrifuged, washed and dried again to obtain the final product iron trihydroxyphosphate.
[0028] The morphology of the prepared ferric trihydroxyphosphate is as follows figure 1 As shown in the figure, we ...
Embodiment 2
[0030] In this example, FeCl3·6H2O and Na3PO4·12H2O were used as raw materials, and the molar ratio of Fe:P was 1:1 for batching, and hydrothermal method was used to prepare ferric hydroxyphosphate. The details are as follows: Weigh 5.406g of FeCl3·6H2O and dissolve it in a small amount of deionized water, then add 7.602g of Na3PO4·12H2O, after stirring continuously for 20 minutes, add an appropriate amount of deionized water to make the volume of the entire solution 100ml. The prepared solution was transferred to a polytetrafluoroethylene liner, and heated at 180° C. for 24 hours. Then it was centrifuged, washed and dried at 80°C for 2h. The product obtained after drying was redissolved in 100ml of 1mol / L hydrochloric acid solution, stirred continuously for 30 minutes, centrifuged, washed and dried again to obtain the final product iron trihydroxyphosphate.
[0031] The morphology of the prepared ferric trihydroxyphosphate is as follows image 3 As shown, we can observe fro...
Embodiment 3
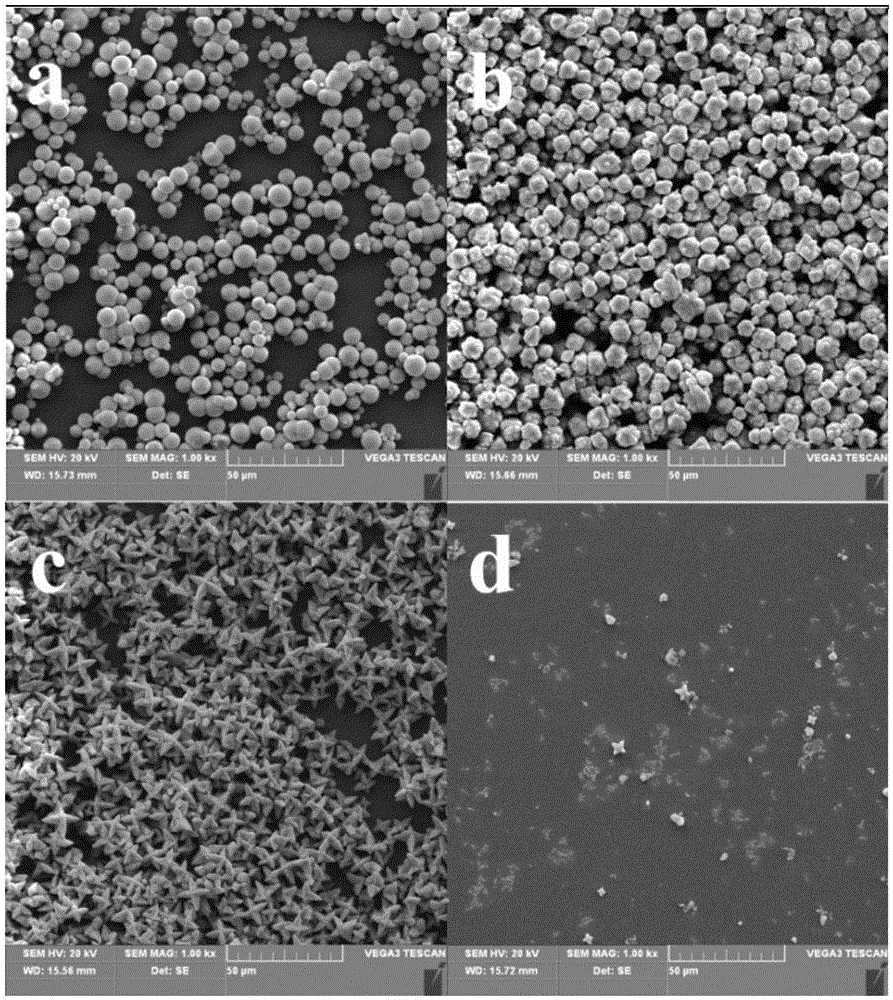
[0033] In this example, FeCl3·6H2O and Na3PO4·12H2O are used as raw materials, and the molar ratio of Fe:P is 1:0.85--1.15 for batching, and the hydrothermal method is used to prepare ferric hydroxyphosphate. The details are as follows: Weigh four parts of 5.406g of FeCl3·6H2O and dissolve them in a small amount of deionized water respectively, then add 6.462g, 7.222g, 7.983g, and 8.743g of Na3PO4·12H2O respectively, and after stirring continuously for 20 minutes, add an appropriate amount of deionized water Ionized water, so that the total solution volume is 100ml. The prepared solution was transferred to a polytetrafluoroethylene liner, and heated at 140° C. for 24 hours. Then it was centrifuged, washed and dried at 80°C for 2h. The product obtained after drying was redissolved in 100 ml of 1 mol / L hydrochloric acid solution, stirred continuously for 30 min, centrifuged, washed and dried again to obtain the final product.
[0034] The morphology of the prepared ferric hydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com