Method for preparing zinc sulfide nanospheres
A technology of zinc sulfide and nanospheres, which is applied in the direction of zinc sulfide and nanotechnology, which can solve the problems of difficult control of product size and shape, harsh high-temperature reaction conditions, and complicated preparation process, and achieve simple equipment, good stability, and easy operation. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
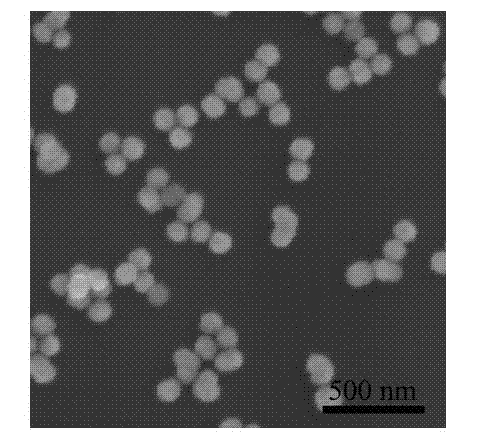
Embodiment 1
[0027] The preparation method of zinc sulfide nanospheres of the present embodiment comprises the following steps:
[0028] 1) Dissolve 0.22 g zinc acetate and 0.05 g polyvinylpyrrolidone in 100 ml deionized water to make solution A; weigh 0.075 g thioacetamide and dissolve it in 50 ml deionized water to make solution B;
[0029] 2) Mix 100 ml of solution A and 50 ml of solution B as a reaction solution, and put the reaction solution in a water bath at 90 °C for 2 hours to obtain zinc sulfide nanospheres;
[0030] 3) After the water bath reaction, centrifuge the suspended reaction solution, and then wash the precipitate with deionized water and absolute ethanol;
[0031] 4) Dry the precipitate in a vacuum drying oven at 50°C for 5 hours to obtain white zinc sulfide powder.
[0032] A certain amount of surfactant polyvinylpyrrolidone is added to the zinc acetate solution, the purpose is to control the morphology of the product, and also to prevent particle agglomeration; the p...
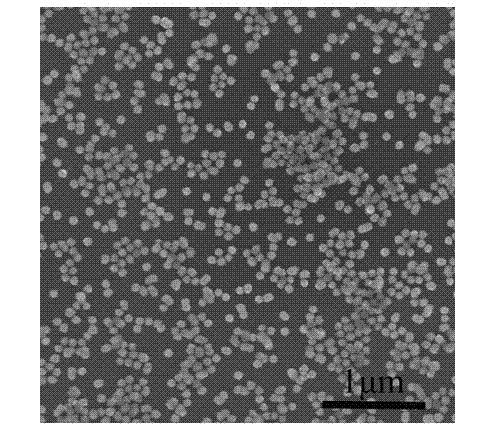
Embodiment 2
[0034] The preparation method of zinc sulfide nanospheres of the present embodiment comprises the following steps:
[0035] 1) Dissolve 0.22 g zinc acetate and 0.15 g polyvinylpyrrolidone in 100 ml deionized water to make solution A; weigh 0.075 g thioacetamide and dissolve it in 50 ml deionized water to make solution B;
[0036] 2) Mix 100 ml of solution A and 50 ml of solution B as a reaction solution, and put the reaction solution in a water bath at 90 °C for 2 hours to obtain zinc sulfide nanospheres;
[0037] 3) After the water bath reaction, centrifuge the suspended reaction solution, and then wash the precipitate with deionized water and absolute ethanol;
[0038] 4) Dry the precipitate in a vacuum drying oven at 50°C for 5 hours to obtain white zinc sulfide powder.
[0039] A certain amount of surfactant polyvinylpyrrolidone is added to the zinc acetate solution, the purpose is to control the morphology of the product, and also to prevent particle agglomeration; the p...
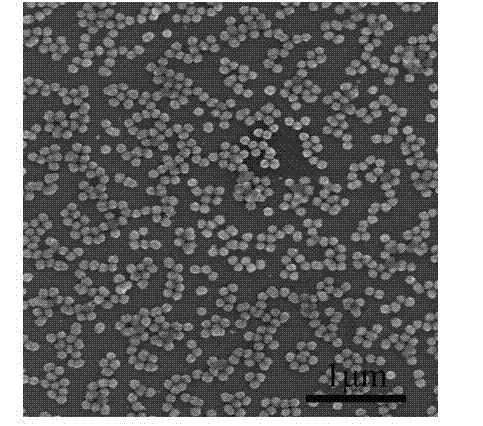
Embodiment 3
[0041] The preparation method of zinc sulfide nanospheres of the present embodiment comprises the following steps:
[0042] 1) Dissolve 0.22 g zinc acetate and 0.15 g polyvinylpyrrolidone in 100 ml deionized water to make solution A; weigh 0.075 g thioacetamide and dissolve it in 50 ml deionized water to make solution B;
[0043] 2) Mix 100 ml of solution A and 50 ml of solution B as a reaction solution, put the reaction solution in a water bath at 90 °C for 4 hours to obtain zinc sulfide nanospheres;
[0044] 3) After the water bath reaction, centrifuge the suspended reaction solution, and then wash the precipitate with deionized water and absolute ethanol;
[0045] 4) Dry the precipitate in a vacuum drying oven at 60°C for 4 hours to obtain white zinc sulfide powder.
[0046] A certain amount of surfactant polyvinylpyrrolidone is added to the zinc acetate solution, the purpose is to control the morphology of the product, and also to prevent particle agglomeration; the purpo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com