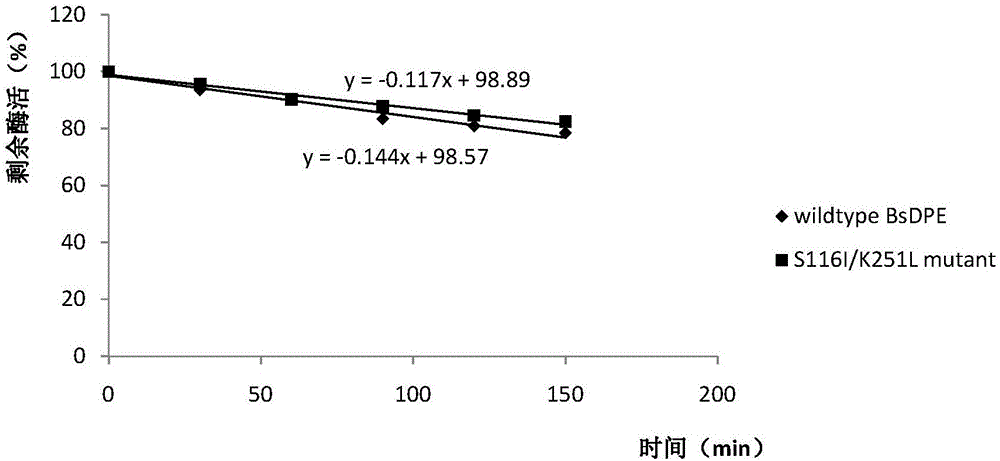
High-thermal-stability mutant of D-allulose-3-epimerase and application thereof
A technology of epimerase and high thermal stability, applied in the field of genetic engineering, can solve problems such as unfavorable industrial application and promotion of D-psicose, and achieve the effects of improving thermal stability and prolonging the service life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1. Establishment of a mutant library of D-psicose-3-epimerase
[0023] The BsDPE-pET29a (+) plasmid synthesized with the whole gene (synthesized by Changzhou Jiyu Biotechnology Co., Ltd., the nucleotide shown in SEQ ID NO.2 encoding the amino acid sequence shown in SEQ ID NO.1 is cloned on the plasmid sequence) as a template, using primers F1 and R1 as forward and reverse primers, respectively, to perform error-prone PCR to construct a mutant library.
[0024] The primer sequence is as follows: F1: 5'-GGAATTC CATATG AACAAAGTGGGC-3';R1:5'-CGC GGATCC TTATGCCAGTTTTTC-3', with NdeI and BamHI restriction enzyme sites at both ends.
[0025] The error-prone PCR reaction system is as follows: 10* PCR buffer 2.5μL, 10mM dGTP 0.5μL, 10mM dTTP 0.5μL, 10mM dCTP 2.5μL, 10mM dATP 2.5μL, 1mM MnCl 2 2.5 μL, 55 mM MgCl 2 2.5 μL, 10 μM F11 μL, 10 μM R1 1 μL, Template (10ng / μL) 1 μL, Taq DNA polymerase (5U / μL, takara) 0.2 μL, ddH 2 O make up to 25 μL.
[0026] Error-pron...
Embodiment 2
[0028] Example 2 Activation and induced expression of mutants
[0029] Pick a single clone from the plate cultured overnight to a 96-deep well plate filled with 1 mL of LB liquid medium with a final concentration of 50 μg / mL kanamycin sulfate, and culture overnight at 37°C and 220 rpm with shaking. On the next day, draw 100 μL of the culture solution from the 96 empty plates of the above overnight culture, and add it to a fresh 1 mL 96 deep-well plate containing LB liquid medium with a final concentration of 50 μg / mL kanamycin sulfate, at 37 ° C, 220 rpm After 4 hours of shaking culture, IPTG with a final concentration of 1 mM was added for induction, and then culture was continued at 30° C. for 20 hours. A total of 400 single clones were picked for activity screening.
[0030] Centrifuge the induced bacterial solution at 4°C and 4000 rpm for 15 min, and discard the supernatant. The cells were suspended in 100 μL of 50 mM sodium phosphate buffer (pH 7.0), added with lysozyme...
Embodiment 3
[0031] Example 3 Establishment of High Performance Liquid Chromatography HPLC High Throughput Screening Method
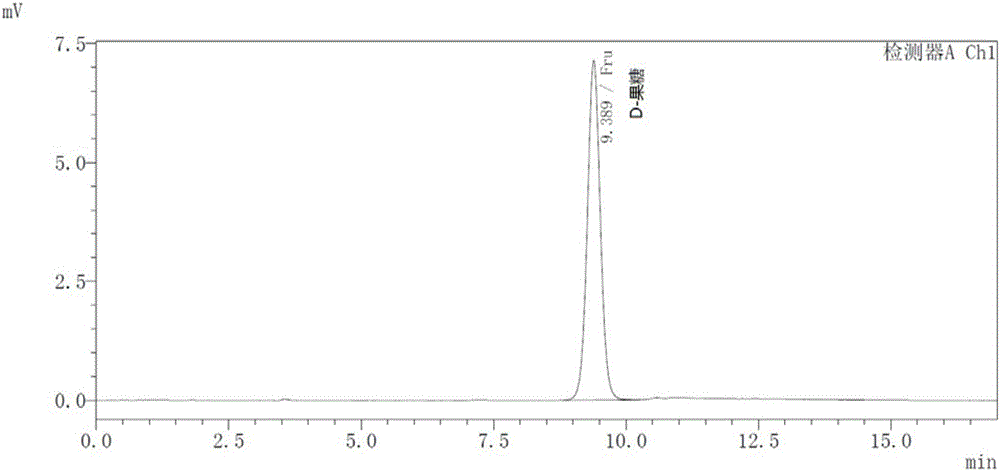
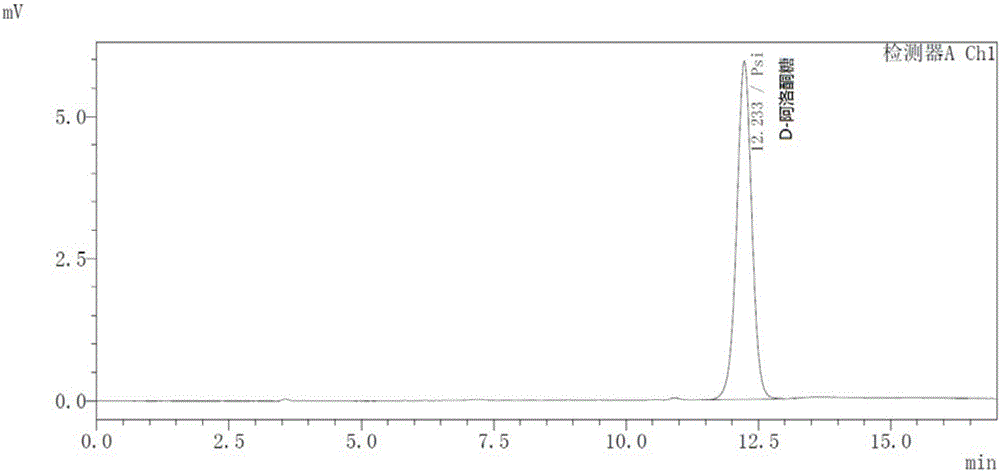
[0032]High performance liquid chromatography HPLC was carried out according to the following conditions: Shimadzu SHIMADZU LC-20A HPLC with RID detectoror equivalent; analytical column: Waters Sugar-Pak I, 6.5×300mm column; mobile phase: water; flow rate: 0.6mL / min; column temperature : 80°C; detector: RID, detector temperature 60°C.
[0033] Pure D-fructose and D-psicose produced by Sigma were used as standard products, and the sample volume was 20 μL. Chromatographic analysis results see figure 1 and 2 , the retention time of D-fructose is 9.389min ( figure 1 ), the retention time of D-psicose is 12.233min ( figure 2 ), the two have a high degree of separation, and can be used as a method for screening enzyme mutants.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com