Veterinary levamisole gel implant and preparation method thereof
A technology of levamisole and levamisole hydrochloride, which is applied in the fields of pharmaceutical formulation, aerosol delivery, liquid delivery, etc., can solve the problems of difficult long-acting preparations, difficult to reduce blood drug concentration, complicated preparation process, etc., and achieve improvement The effects of compliance and comfort, prolonged drug action time, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
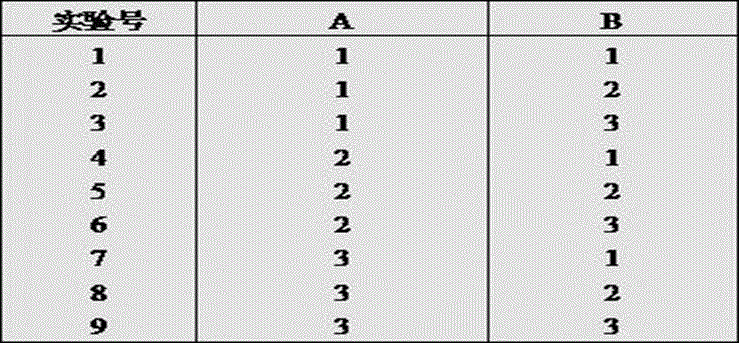
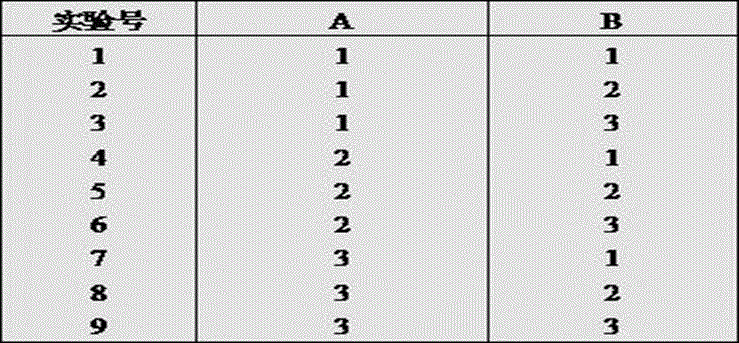
Image
Examples
Embodiment 1
[0025] Embodiment 1: a kind of veterinary levamisole gel implant, is characterized in that: comprise the raw material composition of following parts by weight: 4 grams of levamisole hydrochloride, 10 grams of polylactic acid, 80 grams of benzyl benzoate, poloxane Mu 188 2 grams, monoethanolamine 1 gram, potassium sorbate 10 grams.
[0026] A preparation method for veterinary levamisole gel implant, characterized in that: it has the following steps:
[0027] Step 1), take 70% of the stated amount of benzyl benzoate and put it into a concentrated preparation tank, heat it to 37 degrees Celsius, add the stated amount of polylactic acid while stirring, and keep warm for 12 hours at 37 degrees Celsius after adding polylactic acid Hour, while keeping warm, stir, obtain mixed solution 1;
[0028] Step 2), take 15% of the said amount of benzyl benzoate and put it into a clean stainless steel bucket, take the said amount of levamisole hydrochloride and add it to 15% of the amount of b...
Embodiment 2
[0062] Embodiment 2: a kind of veterinary levamisole gel implant, is characterized in that: comprise the raw material composition of following parts by weight: 2 grams of levamisole hydrochloride, 8 grams of polylactic acid, 70 grams of benzyl benzoate, poloxa Mu 188 0.01 grams, monoethanolamine 0.01 grams, potassium sorbate 5 grams.
Embodiment 3
[0063] Embodiment 3: a kind of veterinary levamisole gel implant, is characterized in that: comprise the raw material composition of following parts by weight: 20 grams of levamisole hydrochloride, 80 grams of polylactic acid, 700 grams of benzyl benzoate, poloxa Mu 188 0.1 grams, monoethanolamine 0.1 grams, potassium sorbate 50 grams.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com