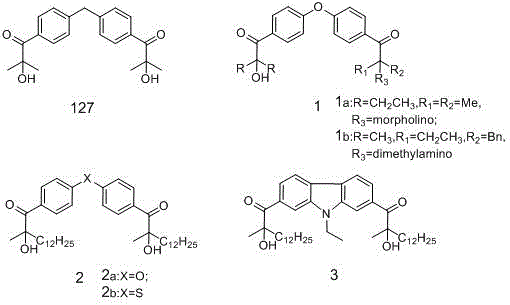
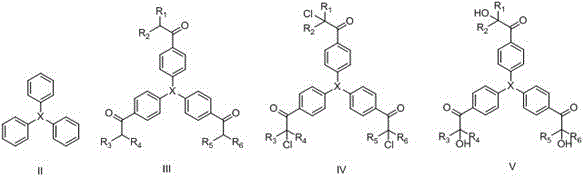
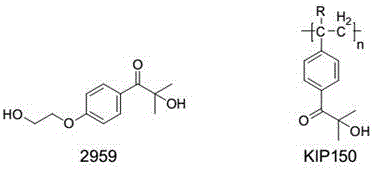
Novel hydroxy-ketone photoinitiator and preparation and application thereof
A technology of photoinitiator and hydroxyketone, which is applied in the field of new hydroxyketone compounds, and can solve problems such as not easy to prepare
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Compound 1 preparation of
[0047] 1) Dissolve triphenylmethane (48.9g, 0.2mol) in 300ml chlorobenzene, control the reaction temperature at 0-10°C, add aluminum trichloride (82.7g, 0.62mol) and stir well, then add isobutyl dropwise Acyl chloride (66.1, 0.62mol), stirring for 2 hours after dropping, stop the reaction, pour the reaction solution into dilute hydrochloric acid made of 800g and 130ml concentrated hydrochloric acid, stir for 0.5h, let stand to separate the organic phase, and extract the water with dichloromethane phase, combined the organic phases, and washed with 200ml saturated sodium bicarbonate solution until the pH value of the organic phase became neutral, dried, and the solvent was recovered by precipitation to obtain a light yellow oil with a purity of 97%. It is also possible not to recover the solvent, and the organic phase is used Dry over anhydrous magnesium sulfate and proceed directly to the next reaction.
[0048] 2) Add chlorine ...
Embodiment 2
[0051] Example 2: Compound 2 preparation of
[0052] 1) Dissolve triphenylphosphine (52.5g, 0.2mol) in 300ml chlorobenzene, control the reaction temperature at 0-10°C, add aluminum trichloride (82.7g, 0.62mol) and stir well, then add isobutyl dropwise Acyl chloride (66.1, 0.62mol), after dropping, stir and react for 2 hours, stop the reaction, pour the reaction solution into dilute hydrochloric acid made of 800g and 130ml concentrated hydrochloric acid, stir for 0.5h, let stand to separate the organic phase, and extract with dichloromethane Water phase, combined organic phase, and washed with 200ml saturated sodium bicarbonate solution until the pH value of the organic phase became neutral, dried, and the solvent was recovered by precipitating to obtain a light yellow oil with a purity of 95%. It is also possible not to recover the solvent, and the organic phase After drying with anhydrous magnesium sulfate, proceed directly to the next reaction.
[0053] 2) Add chlorine ga...
Embodiment 3
[0057] Example 3: Compound 3 preparation of
[0058] 1) Dissolve triphenylphosphine (52.5g, 0.2mol) in 300ml chlorobenzene, control the reaction temperature at 0-10°C, add aluminum trichloride (82.7g, 0.62mol) and stir well, then add isobutyl dropwise Acyl chloride (66.1, 0.62mol), after dropping, stir and react for 2 hours, stop the reaction, pour the reaction solution into dilute hydrochloric acid made of 800g and 130ml concentrated hydrochloric acid, stir for 0.5h, let stand to separate the organic phase, and extract with dichloromethane Water phase, combined organic phase, and washed with 200ml saturated sodium bicarbonate solution until the pH value of the organic phase became neutral, dried, and the solvent was recovered by precipitating to obtain a light yellow oil with a purity of 96%. It is also possible not to recover the solvent, and the organic phase After drying with anhydrous magnesium sulfate, proceed directly to the next reaction.
[0059] 2) Add chlorine ga...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap